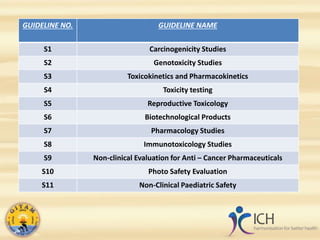
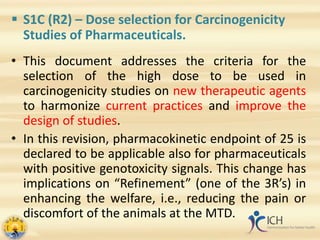
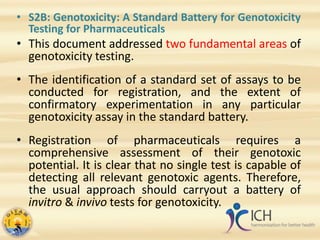
This document summarizes several ICH guidelines related to safety testing of pharmaceuticals. It describes the ICH's purpose of harmonizing drug registration among regulatory authorities and industries. The guidelines cover areas like carcinogenicity studies, genotoxicity testing, toxicokinetics, duration of chronic toxicity studies, reproductive toxicity assessment, safety pharmacology, immunotoxicity, evaluation of anticancer drugs, and photosafety. The summaries provide an overview of the objectives and recommendations within each guideline.