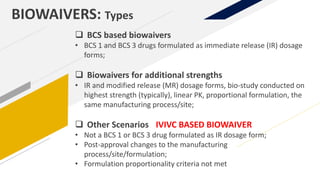
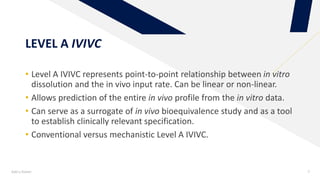
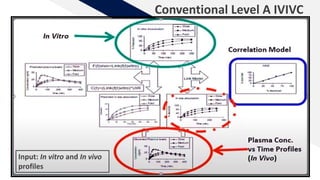
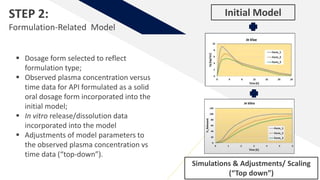
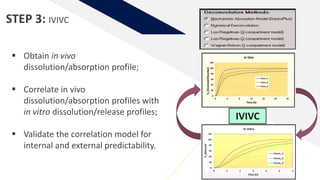
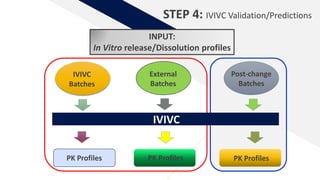
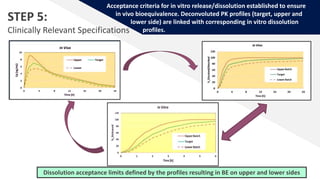
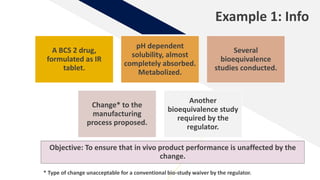
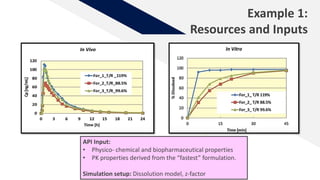
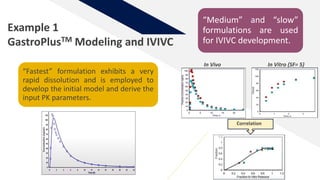
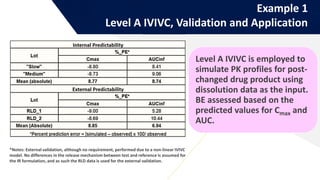
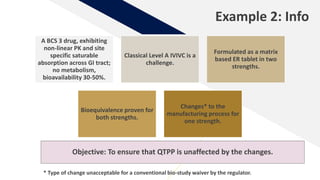
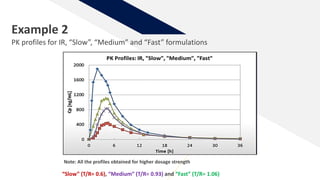
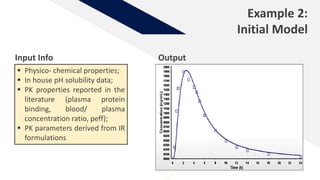
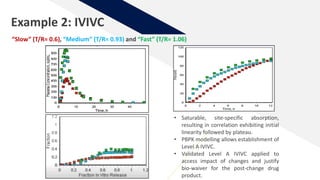
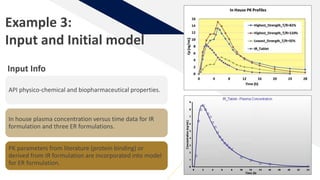
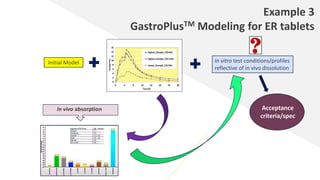
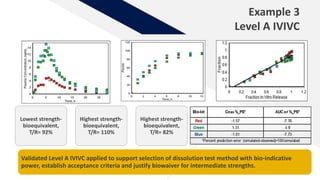
The document discusses the use of in vitro-in vivo correlation (IVIVC) to establish biowaivers for bioequivalence studies in drug development, outlining different levels of correlation and their applications. It provides case studies of three drugs (BCS 2, BCS 3, and BCS 1) demonstrating how IVIVC can be utilized for various formulations and manufacturing changes to predict in vivo performance. The document highlights the advantages of mechanistic physiologically-based pharmacokinetic modeling and challenges faced in IVIVC validation and application.