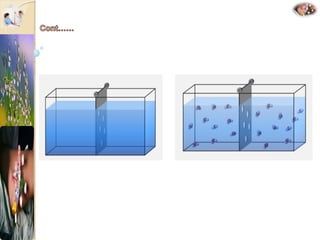
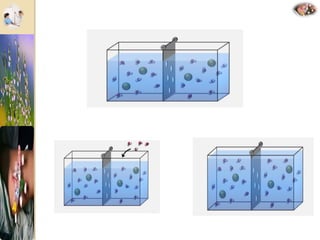
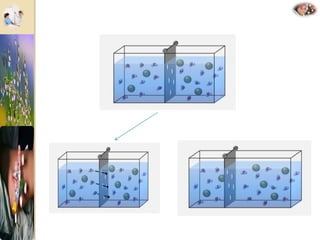
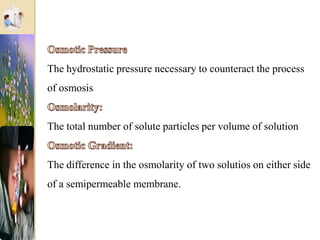
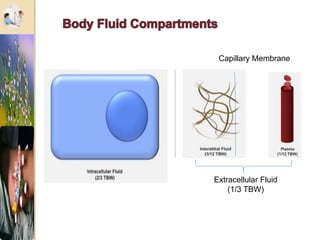
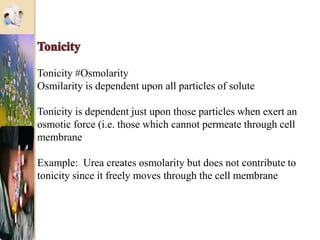
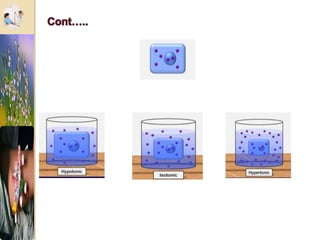
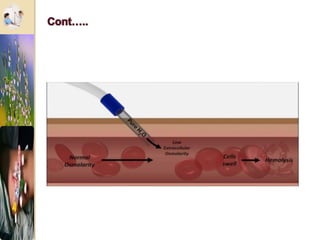
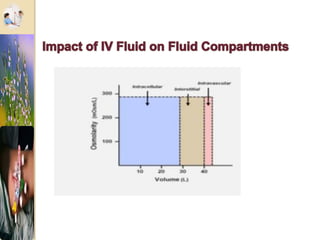
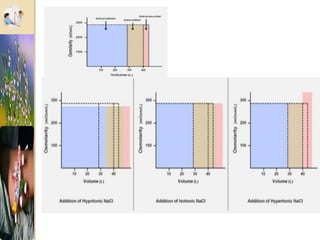
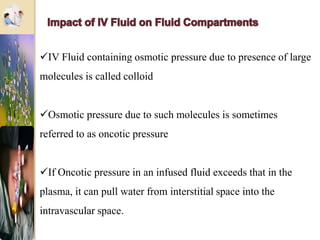
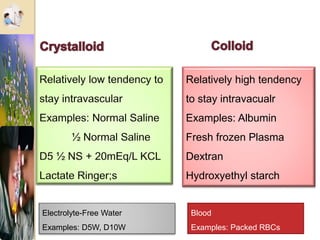
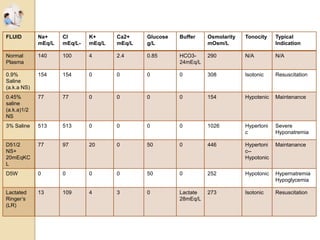
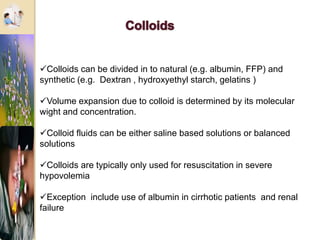
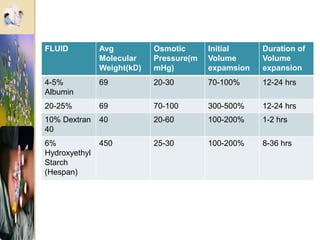
The document discusses the history and uses of intravenous (IV) fluids, describing how IV fluids are used to maintain adequate fluid volume and electrolyte balance in the body. It also explains the concepts of osmosis, tonicity, and body fluid compartments as they relate to IV fluid administration and the goals of IV fluid therapy. Specific types of IV fluids, such as crystalloids and colloids, are categorized and their indications, contraindications, and potential complications are outlined.