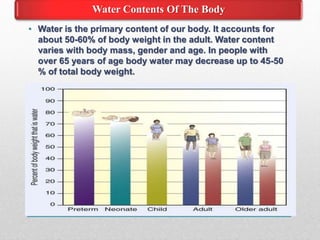
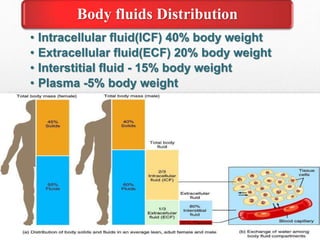
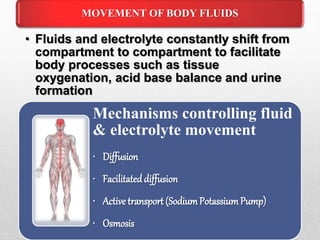
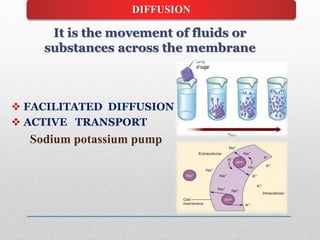
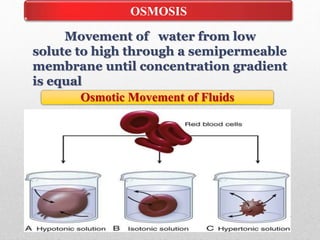
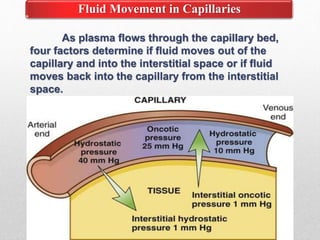
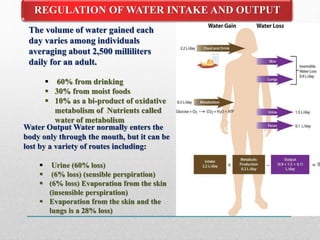
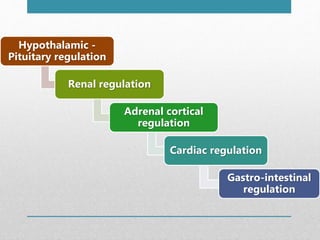
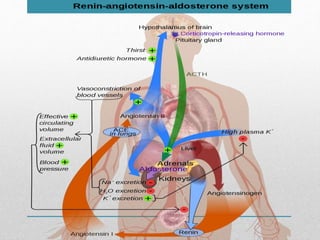
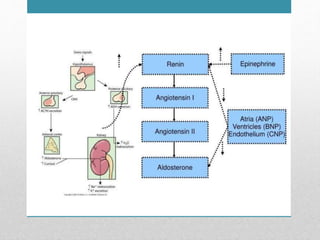
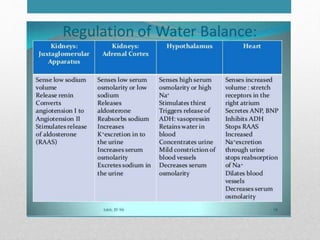
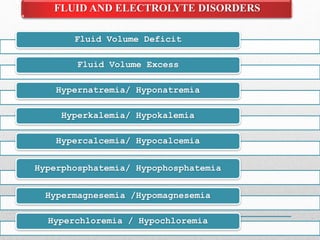
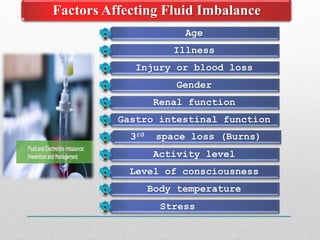
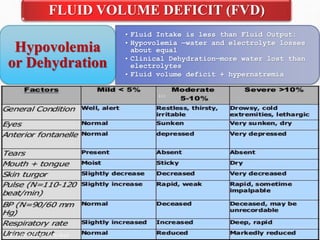
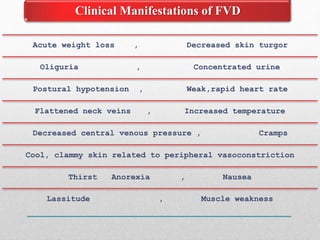
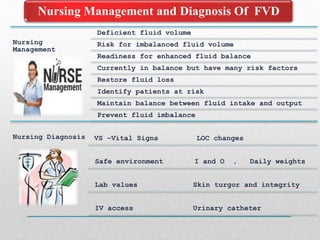
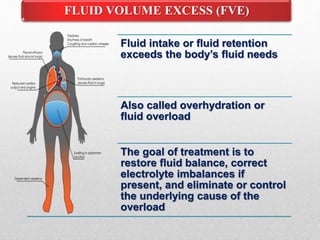
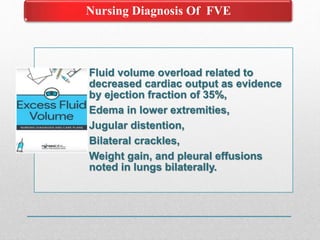
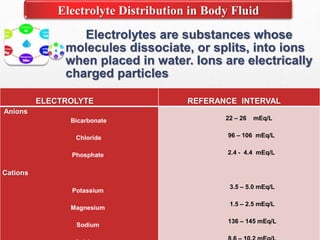
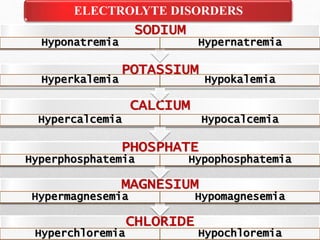
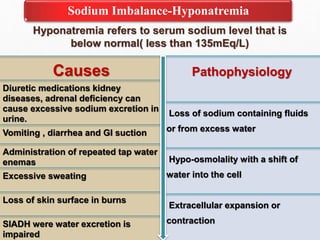
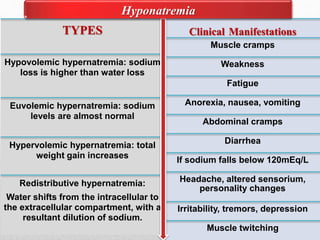
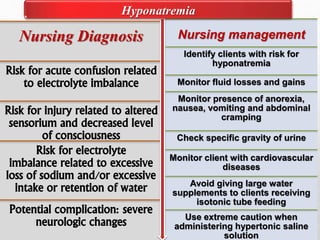
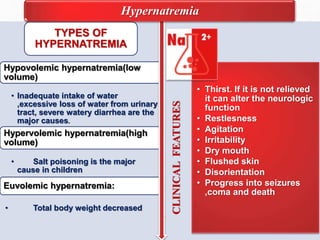
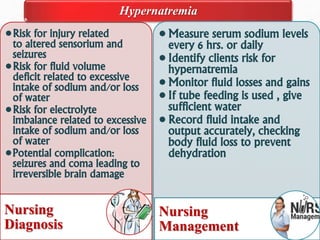
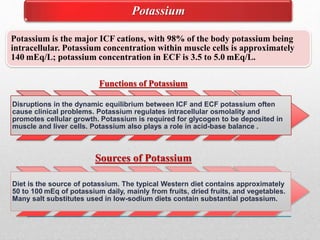
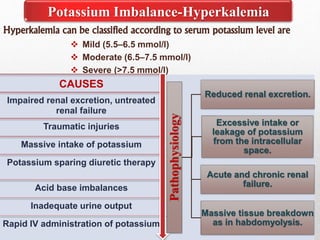
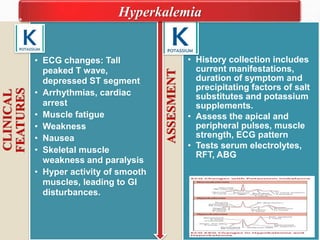
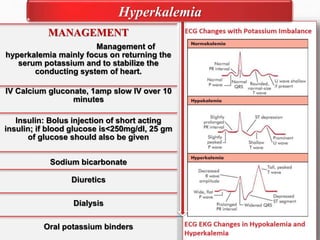
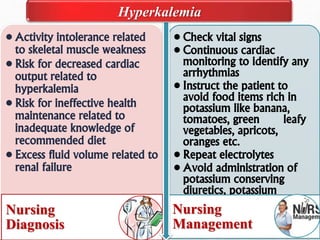
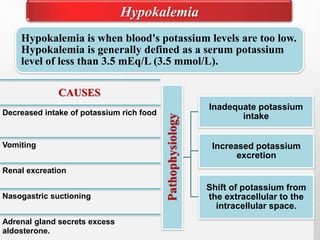
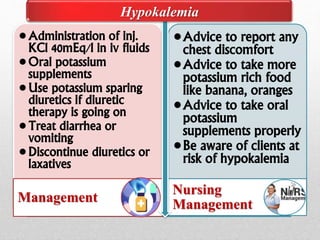
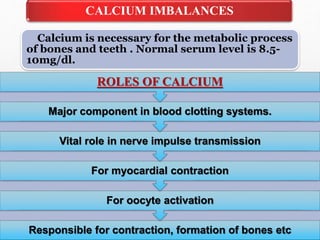
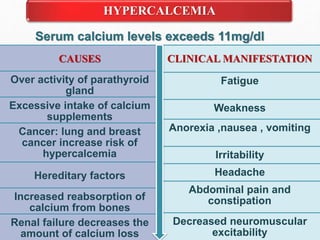
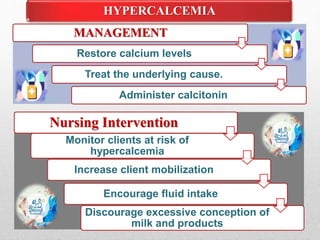
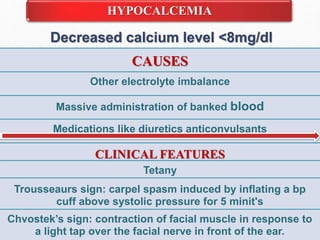
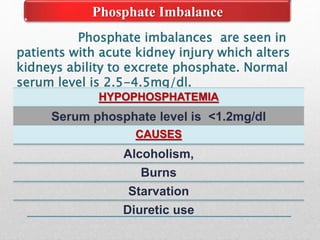
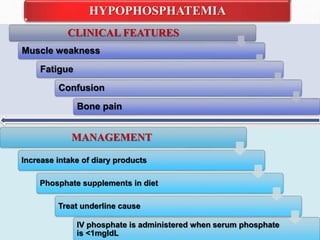
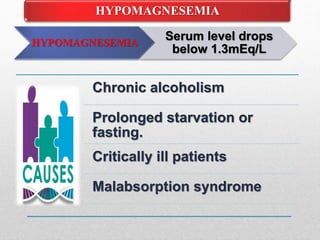
Fluid and electrolyte balance within the body is essential for maintaining health and proper functioning of all body systems. Imbalances can occur when fluid intake exceeds output, leading to fluid volume excess, or when output exceeds intake, resulting in fluid volume deficit. Precise regulation mechanisms aim to keep fluid and electrolytes like sodium, potassium, calcium, and chloride within their normal ranges to support cellular and organ function. Nursing care involves assessing for risk factors, monitoring for signs of imbalance, and treating underlying causes through fluid management, diet, and medication.