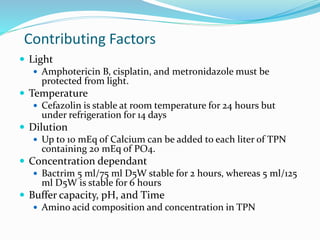
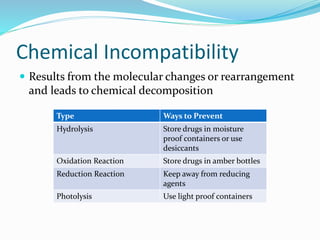
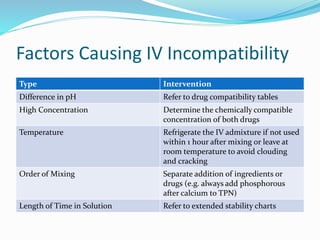
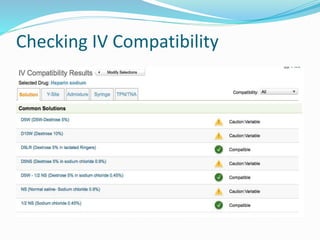
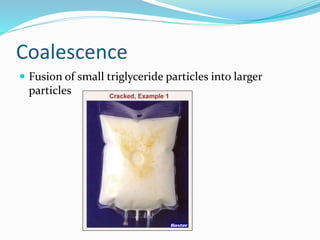
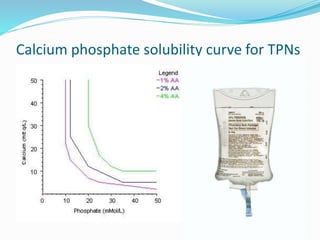
This document defines IV compatibility and incompatibility, discusses where incompatibilities can occur, and provides strategies to prevent them. It describes the different types of incompatibilities including therapeutic, physical, chemical and drug-container incompatibilities. Specific examples are given and factors contributing to incompatibilities like temperature, concentration and pH are explained. Health and financial consequences of incompatibilities are reviewed. The document recommends strategies like checking compatibility references, standardizing protocols, and minimizing mixed drugs to prevent incompatibilities.