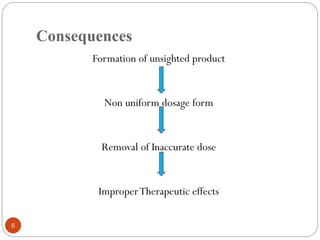
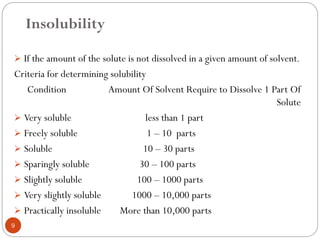
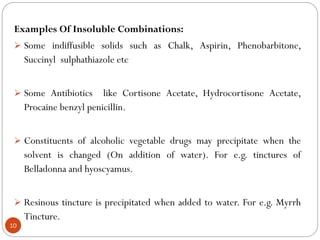
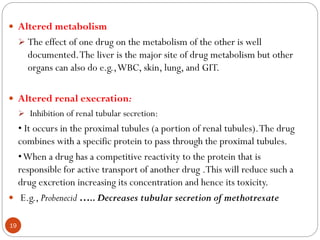
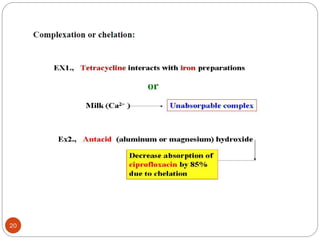
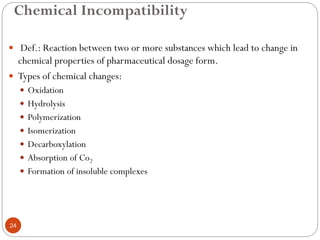
The document discusses various types of pharmaceutical incompatibilities including physical incompatibilities caused by immiscibility, insolubility, or liquefaction between substances and the techniques for overcoming them. It also addresses therapeutic incompatibilities that can occur when certain drugs are prescribed together, exploring mechanisms like pharmacokinetic or pharmacodynamic interactions. Finally, it covers chemical incompatibilities that can happen due to reactions such as oxidation, hydrolysis, or polymerization altering the properties of a dosage form.