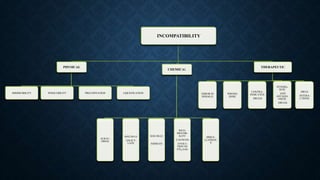
The document discusses drug incompatibility, which occurs when two or more substances produce undesirable effects on pharmaceutical preparations, affecting safety, efficacy, and appearance. It categorizes incompatibility into physical, chemical, and therapeutic types, detailing the causes and examples of each, such as immiscibility, oxidation-reduction reactions, and dosage errors. Understanding these incompatibilities is crucial for ensuring proper drug formulation, dispensing, and administration.