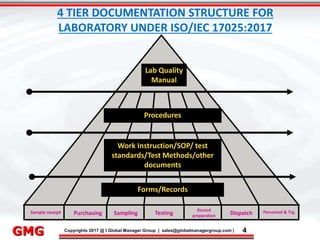
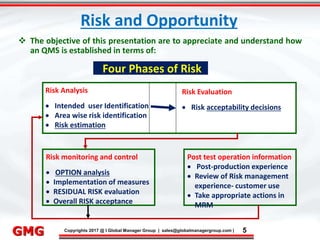
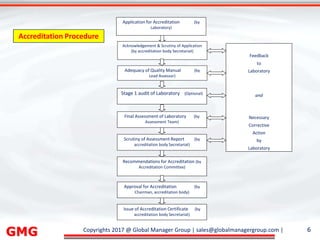
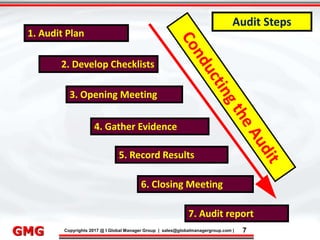
The document discusses the process for updating an existing ISO/IEC 17025:2005 accredited laboratory management system to comply with the revised ISO/IEC 17025:2017 standard. It outlines 8 key steps, including conducting awareness training on the revisions, reviewing and revising documentation, implementing risk-based thinking, training auditors, conducting internal audits, addressing nonconformities, applying for an updated accreditation certificate, and undergoing a final assessment audit. Accredited laboratories must complete the upgrade process within 3 years of the 2017 standard's release.