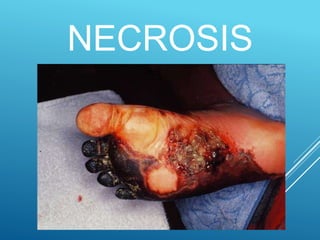
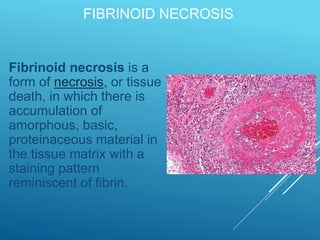
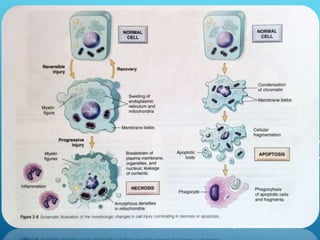
This document discusses irreversible cell injury and the different types of cell death. It begins by introducing the concept that all organ injuries start with alterations at the cellular level. There are two main classes of cell injury - irreversible cell injury, which leads to cell death through necrosis or apoptosis, and reversible cell injury, where cells can survive if the stressor is removed and damage is mild. The two main causes of irreversible injury are an inability to reverse mitochondrial dysfunction and profound disturbances in cell membrane function. Necrosis and apoptosis are then described in more detail, including their morphological changes, types, and differences between the two forms of cell death.