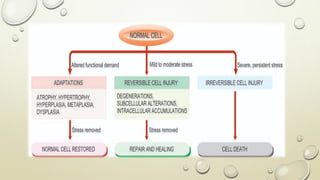
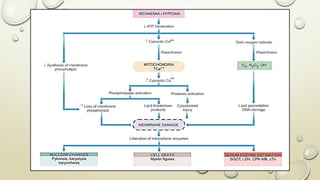
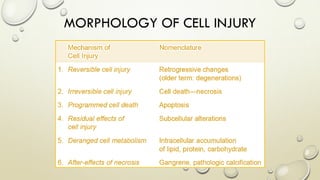
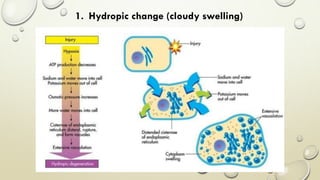
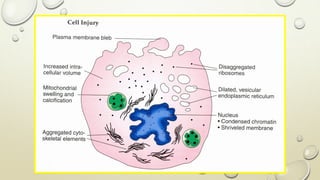
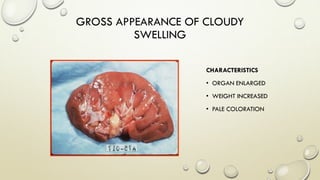
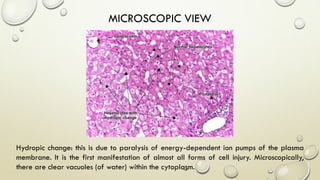
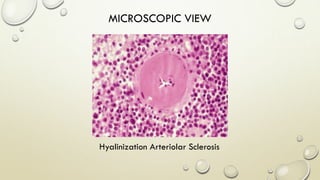
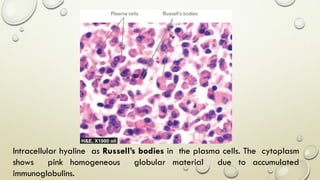
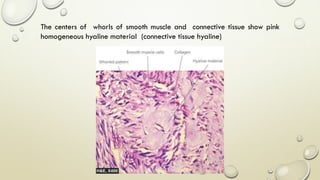
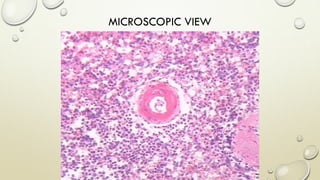
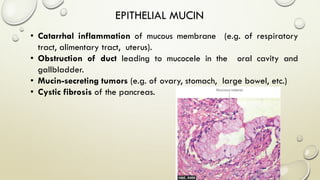
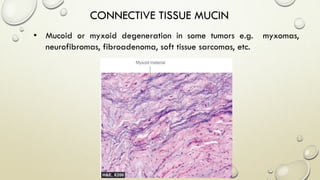
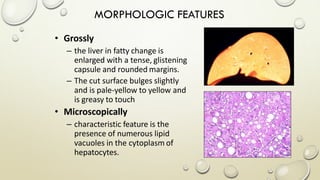
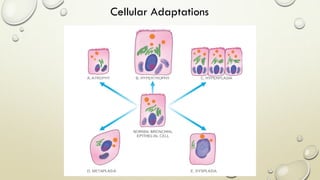
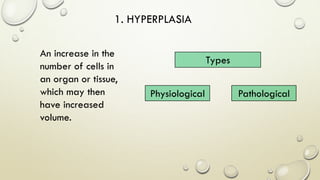
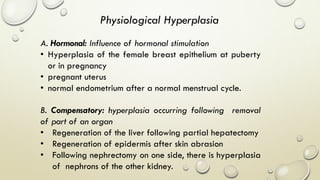
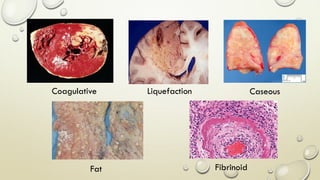
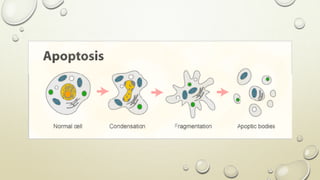
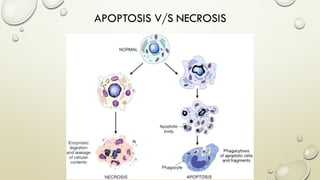
Pathology is the scientific study of disease through examination of tissues and cells. Key terms include:
- Pathology examines structural and functional changes in disease (pathophysiology examines disordered function).
- Disease is a condition causing discomfort, while illness is one's reaction to disease through symptoms and signs.
- Syndromes describe combinations of symptoms from altered physiology. Important tissues include lesions in patients and pathologic changes seen macroscopically and microscopically. Etiology examines causal factors and pathogenesis examines how lesions are produced.