Embed presentation
Download to read offline
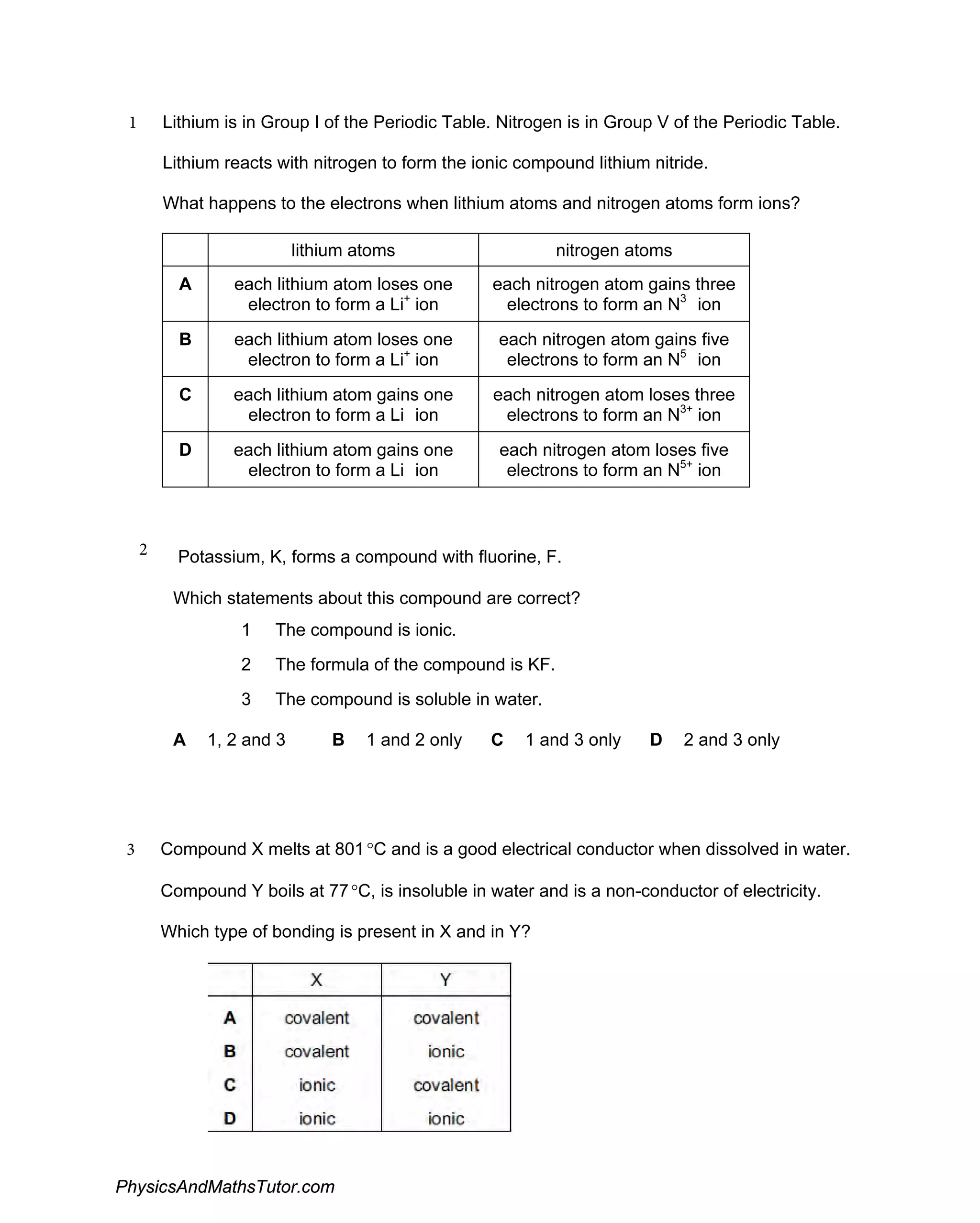

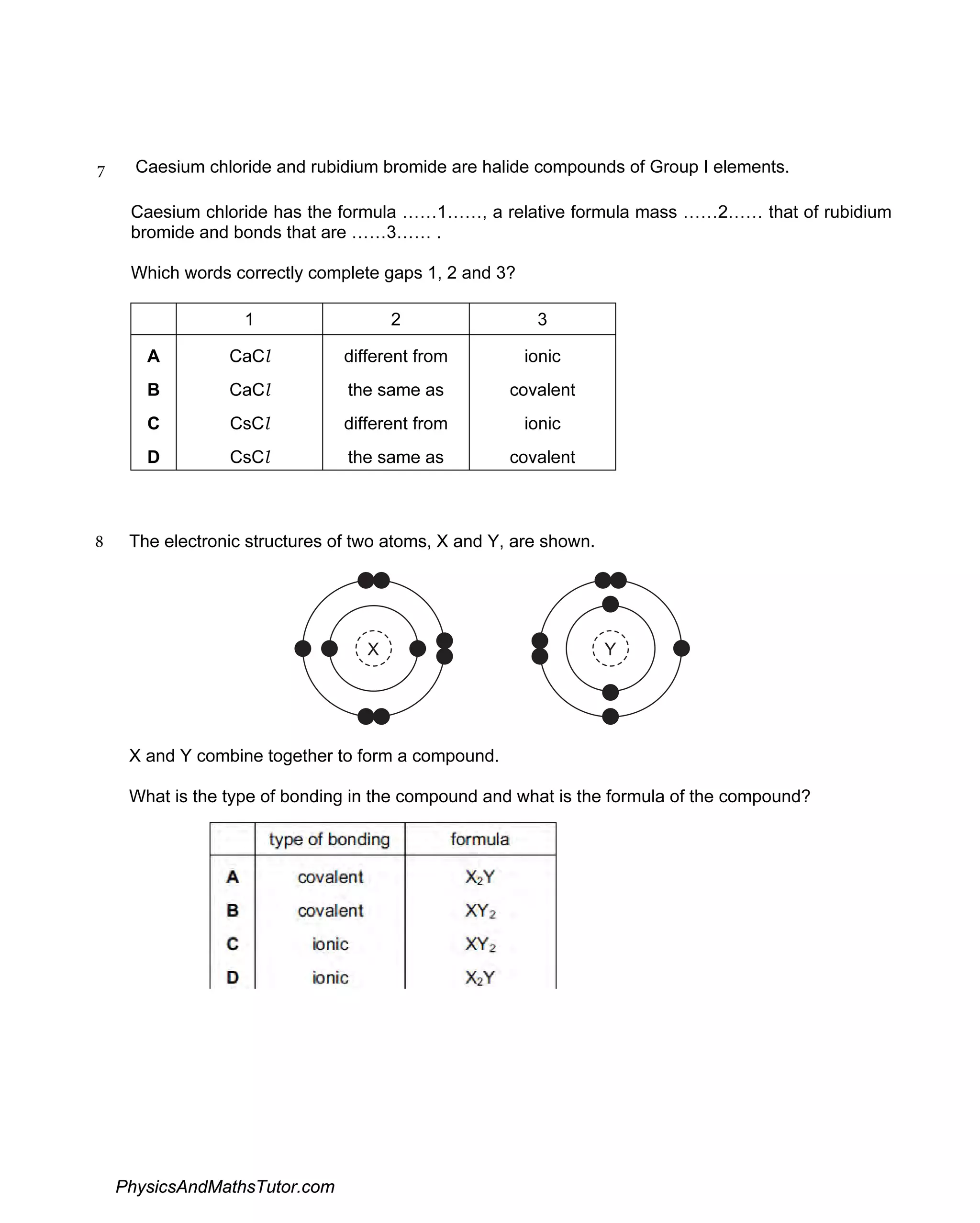
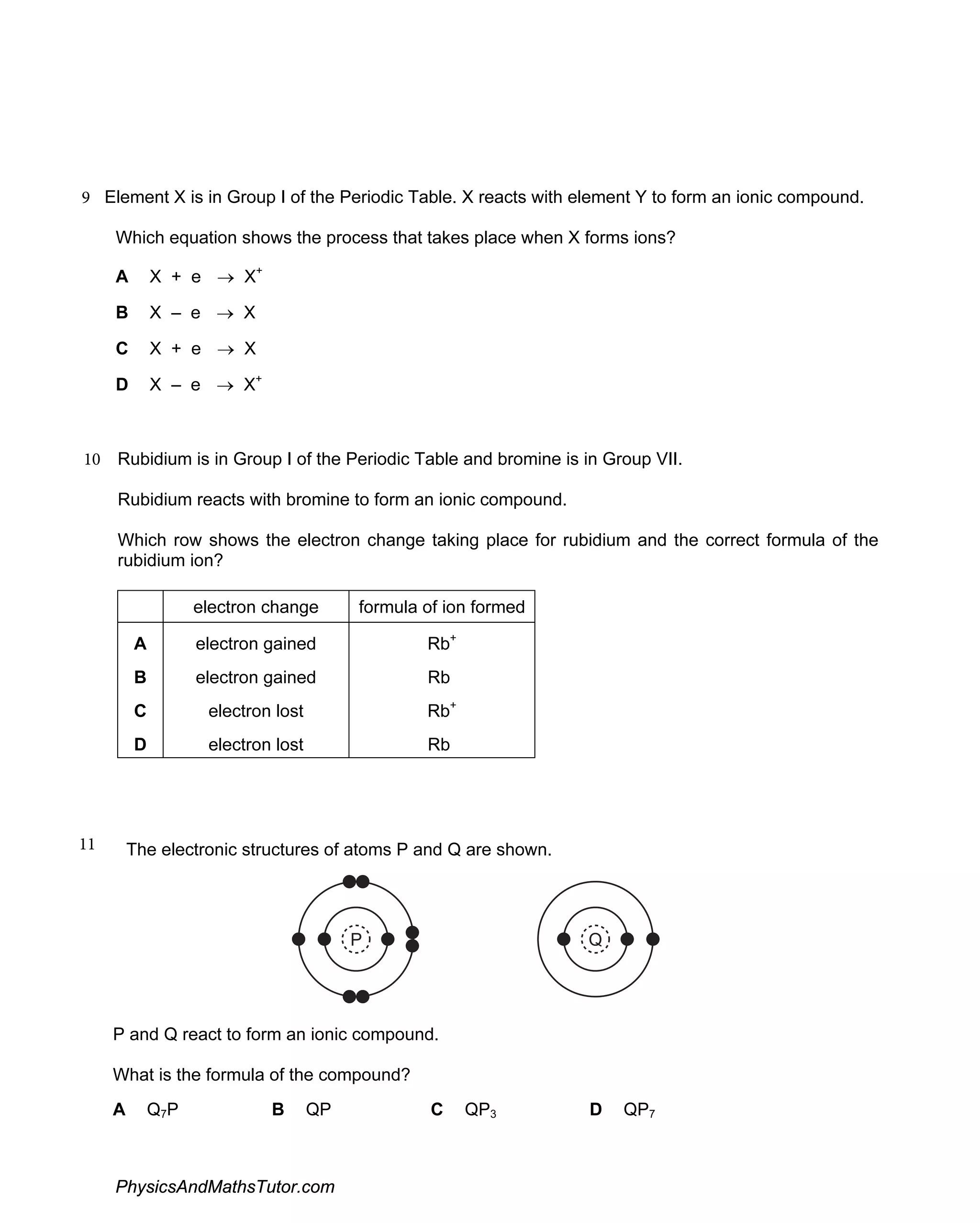
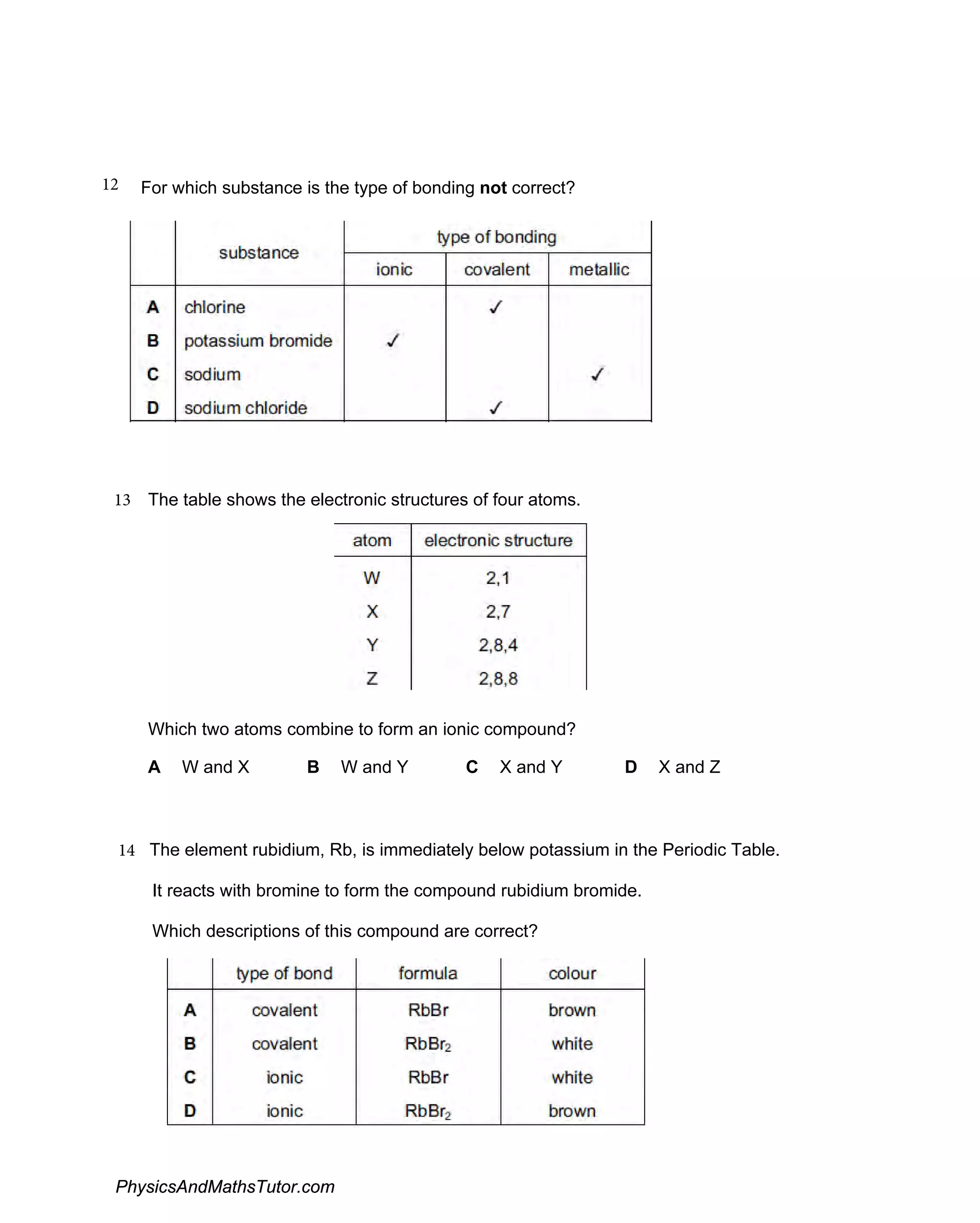
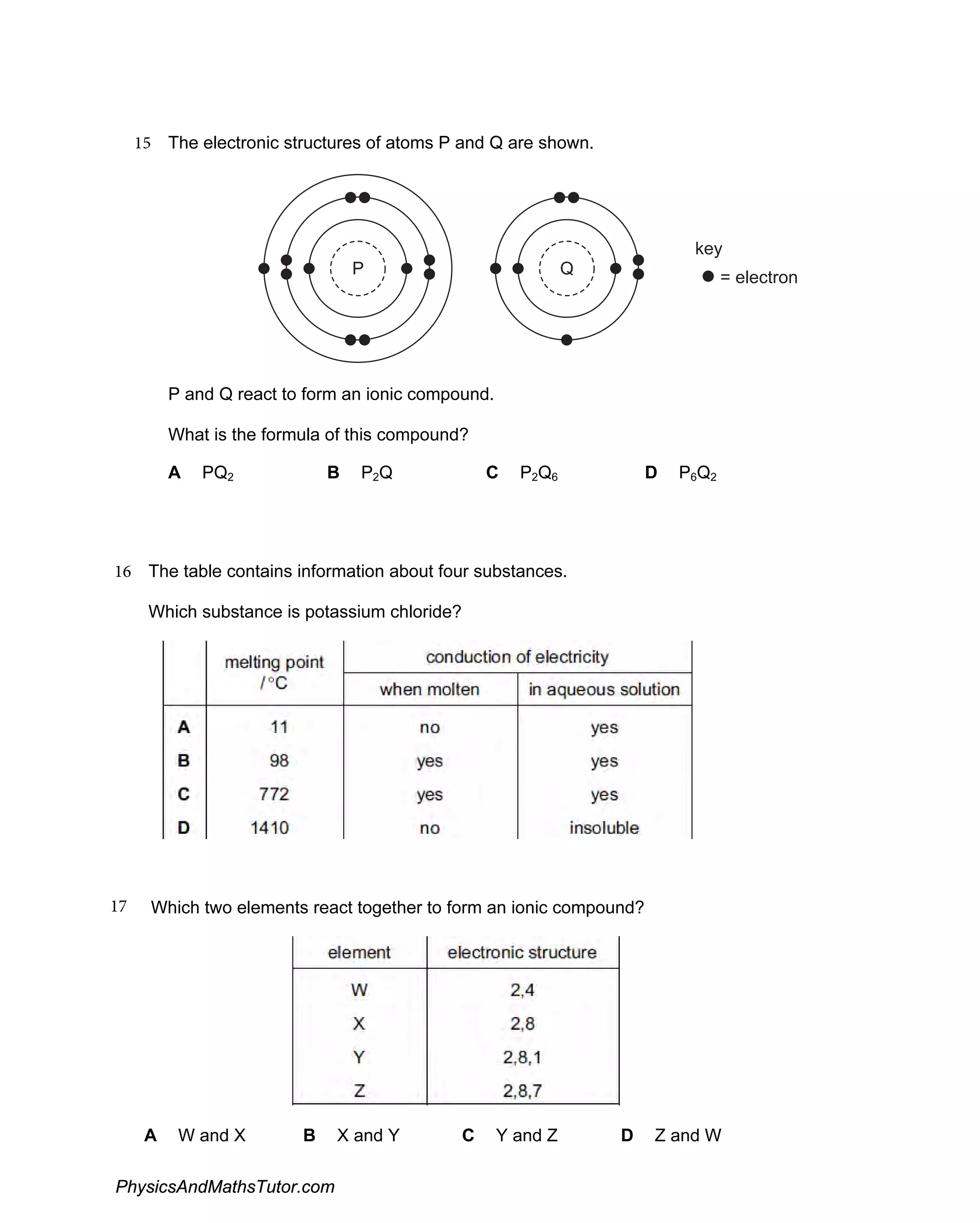

The document discusses ionic bonding and the formation of ions. When lithium and nitrogen react, each lithium atom loses one electron to form a Li+ ion, and each nitrogen atom gains three electrons to form an N3- ion. Rubidium, a Group I element, reacts with bromine, a Group VII element, to form the ionic compound rubidium bromide (RbBr) where each rubidium atom loses one electron to form Rb+ ions. Ionic compounds form when atoms transfer electrons such that the ions have full outer shells and opposite charges, allowing them to be held together by electrostatic attraction.
Discusses the formation of ionic compounds through electron transfer between groups I and V elements, including examples and reactions.
Examines the creation and characteristics of ions in ionic bonding, with equations showcasing electron loss and gain.
Focuses on identifying ionic compounds' properties and how different substances form through ionic bonds.
Covers changes in atoms when forming positive ions and the electronic configuration of ions, including the formation of sodium chloride.






