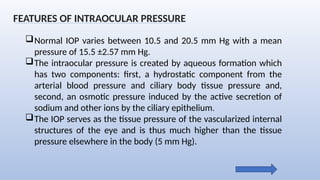
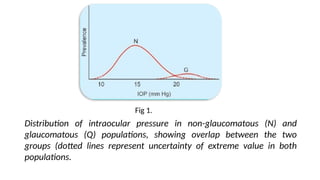
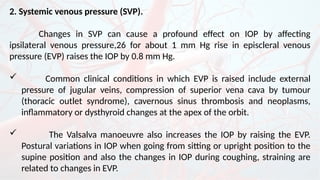
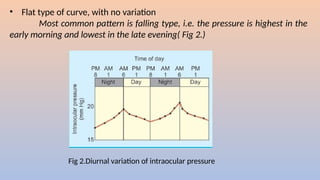
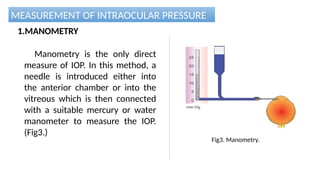
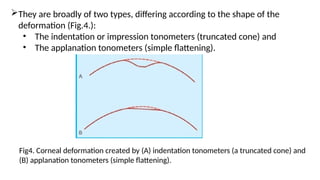
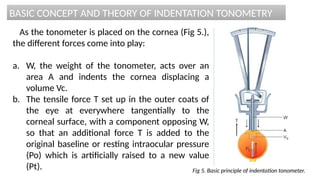
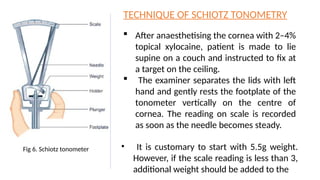
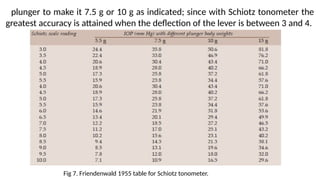
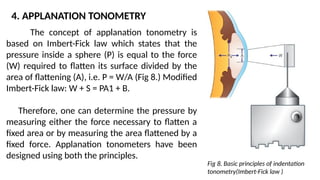
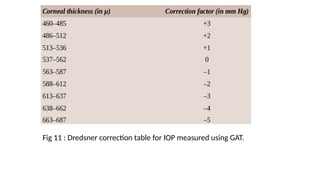
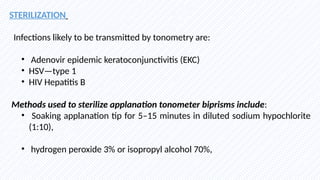
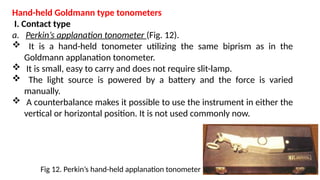
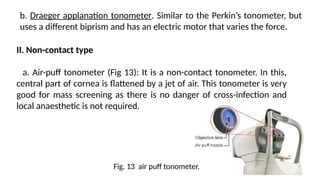
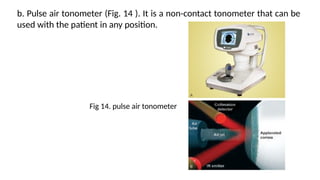
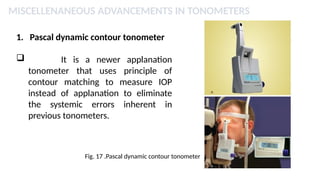
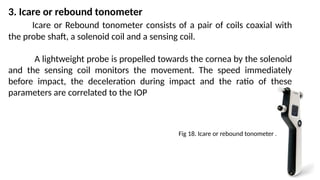
Intraocular pressure (IOP) is the pressure exerted by the contents of the eye, which is crucial for maintaining ocular shape and health. Normal IOP ranges from 10.5 to 20.5 mm Hg, with variations influenced by factors such as age, sex, race, heredity, and environmental conditions. Measurement of IOP is primarily conducted through tonometry, with various methods available, including indentation and applanation techniques.