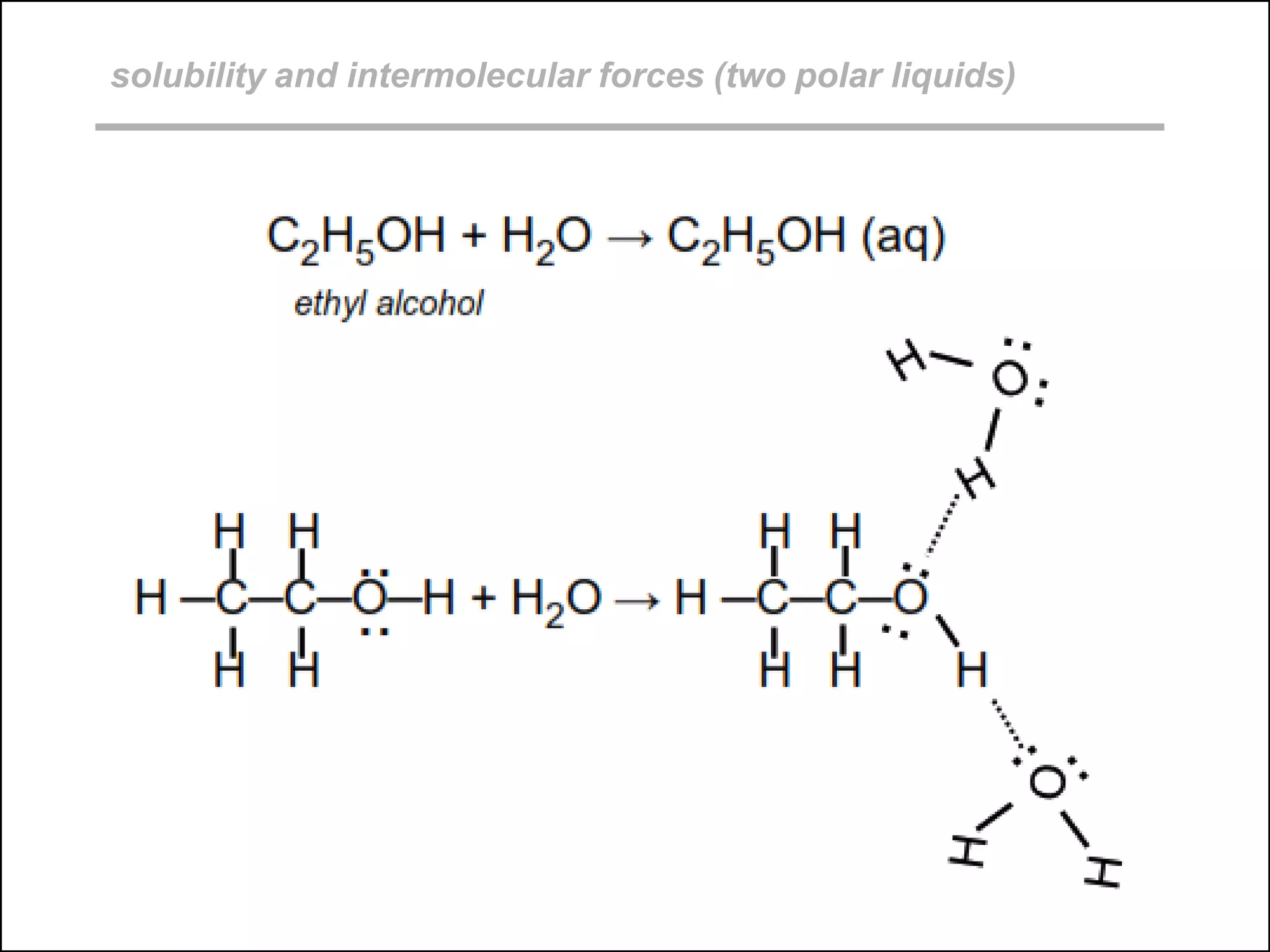
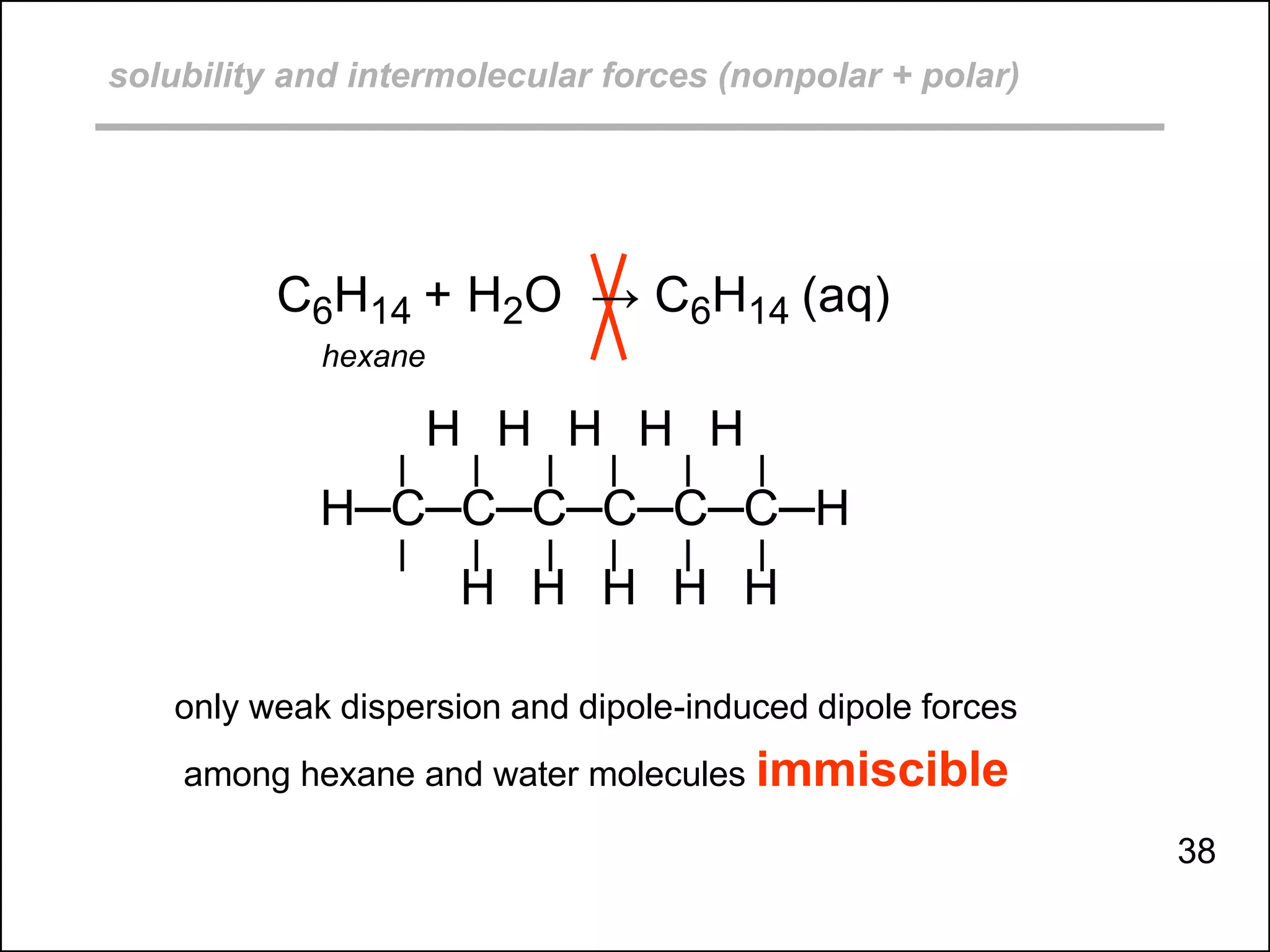
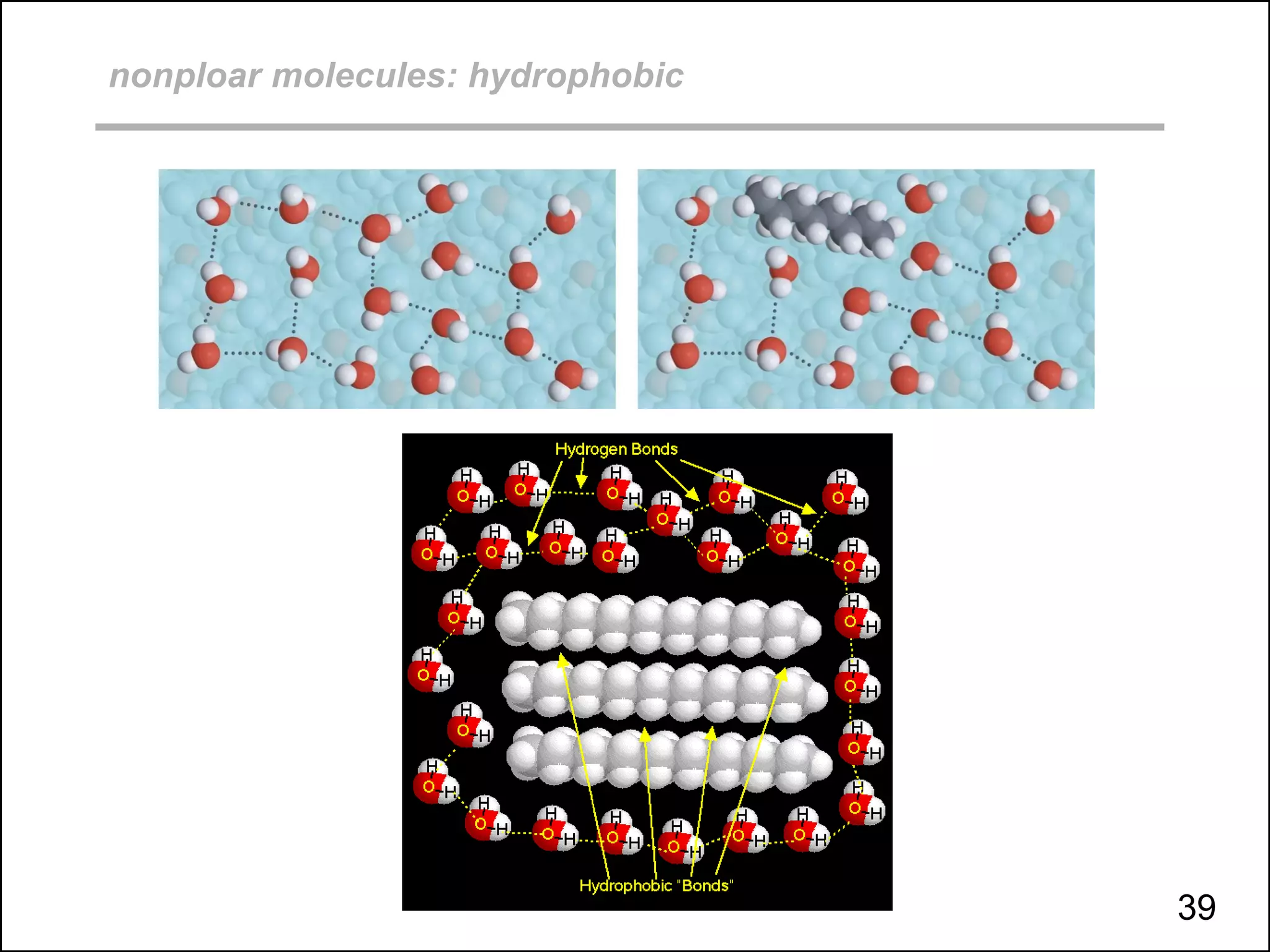
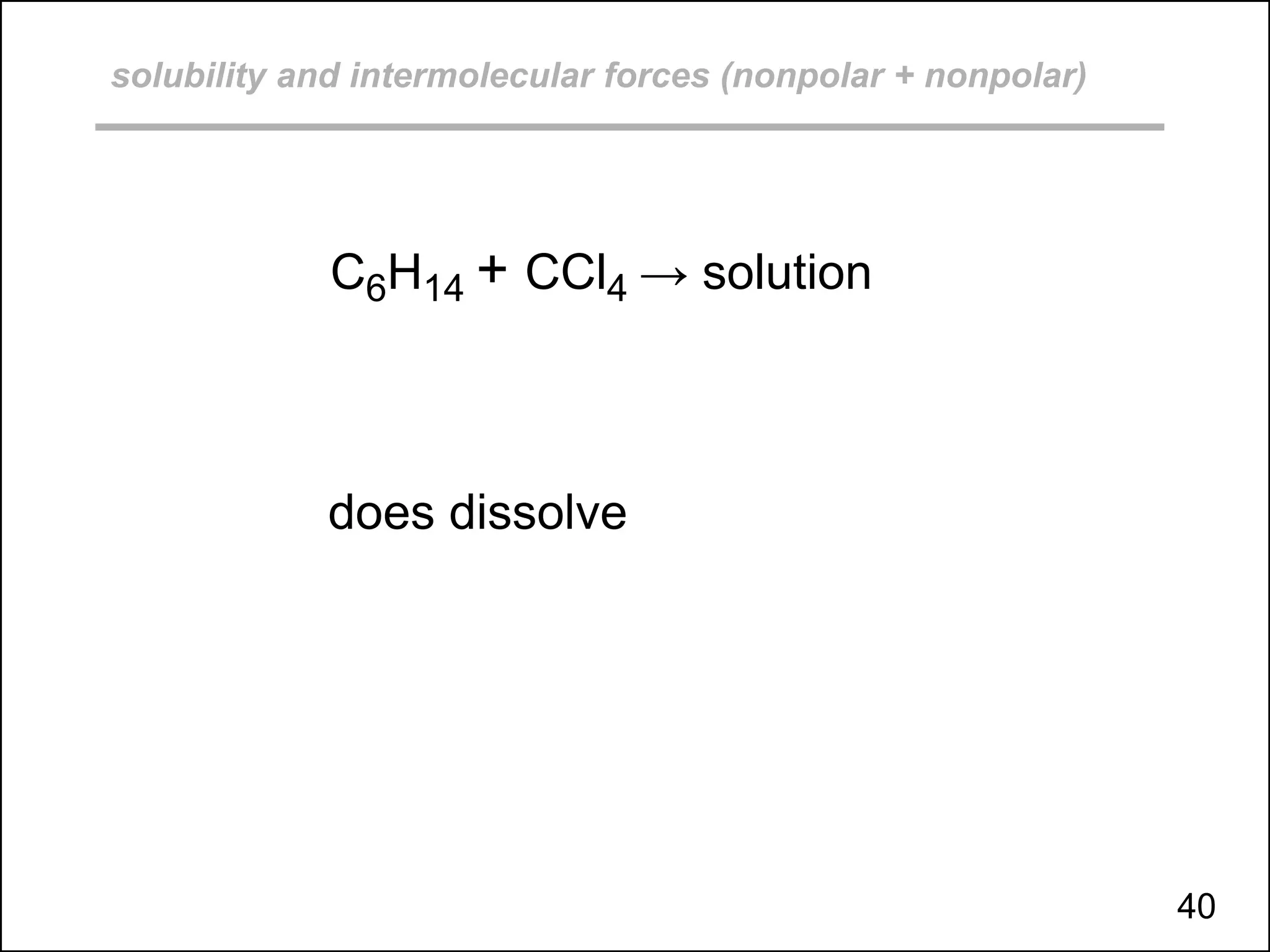
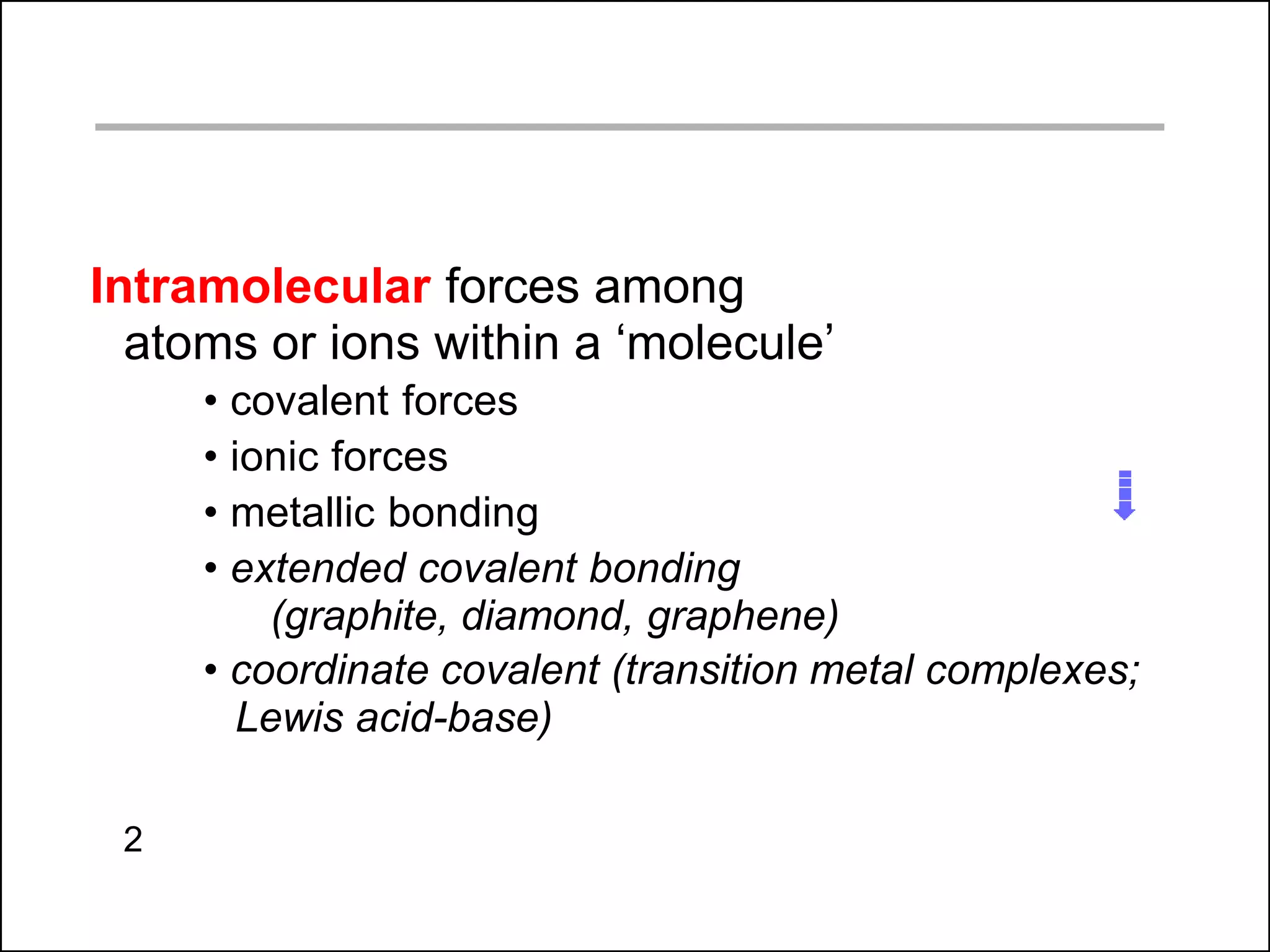
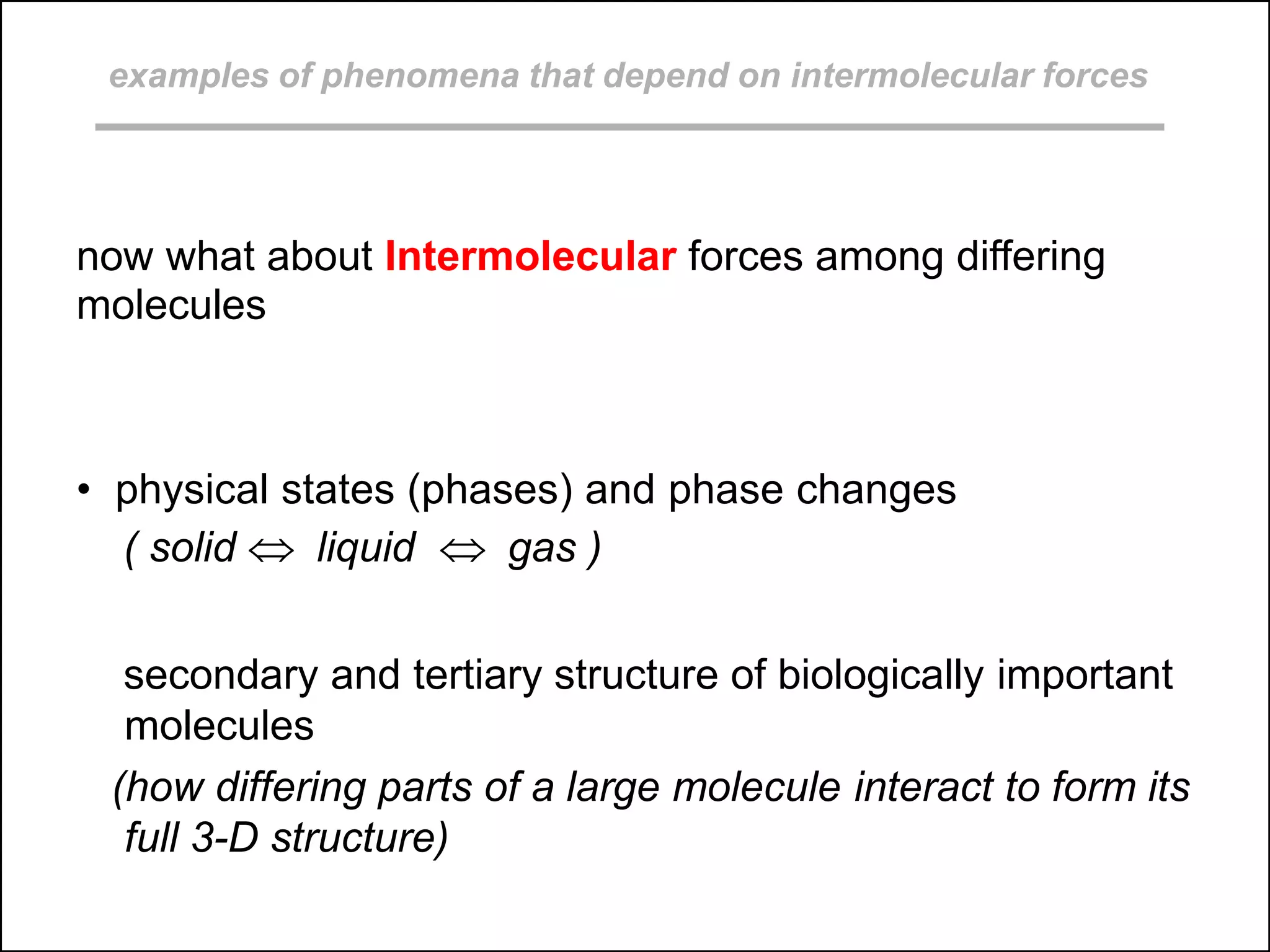
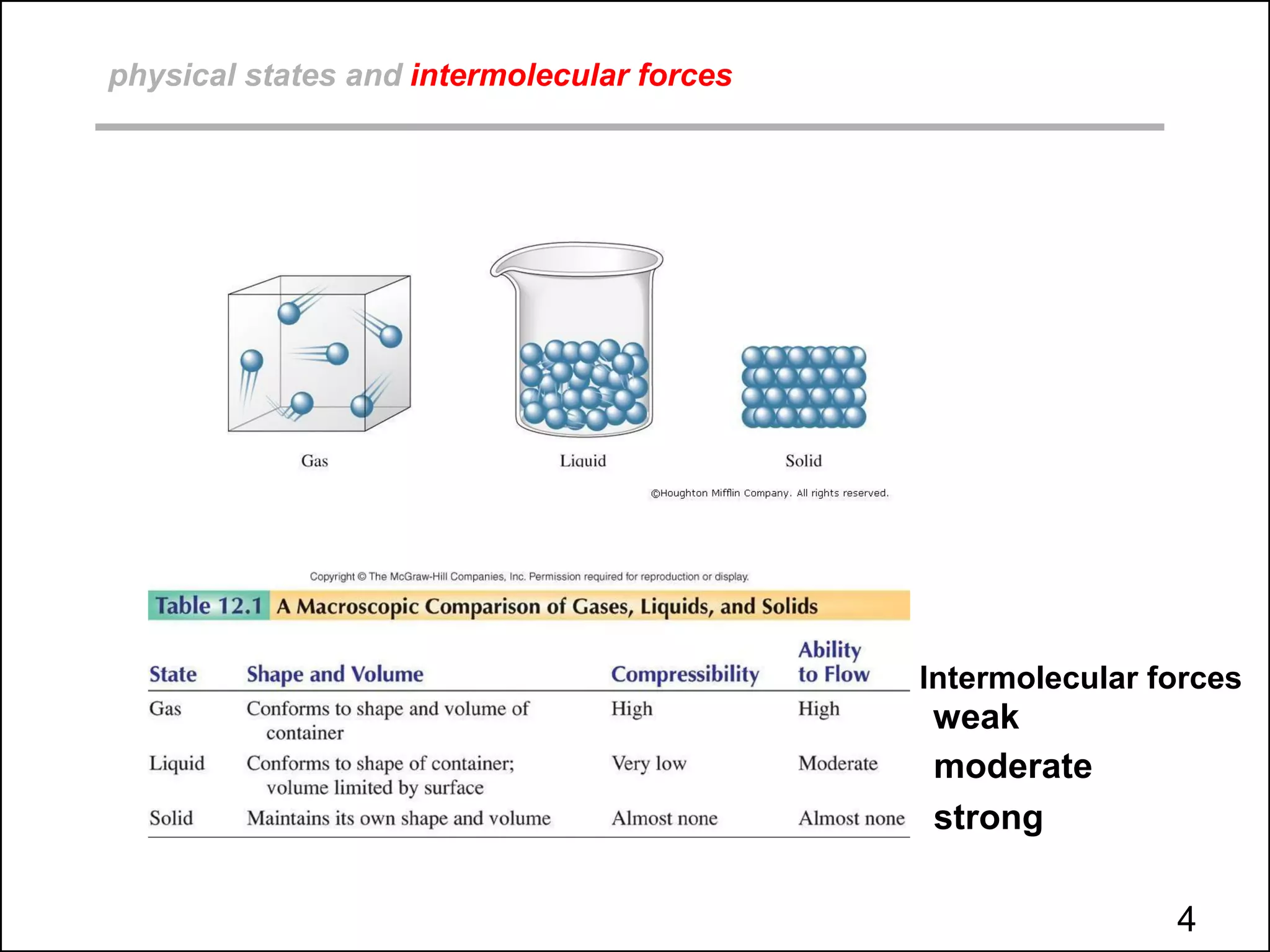
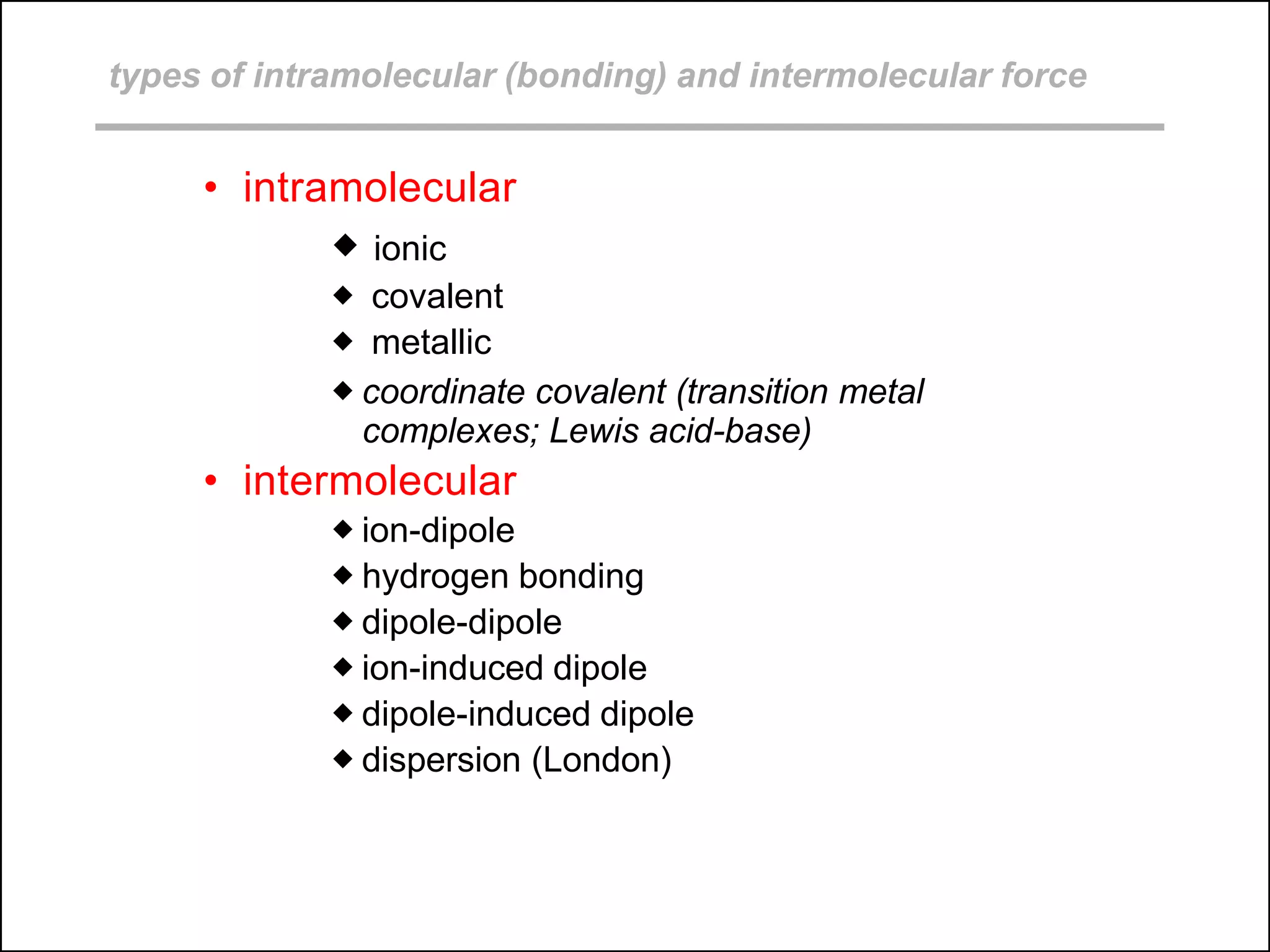
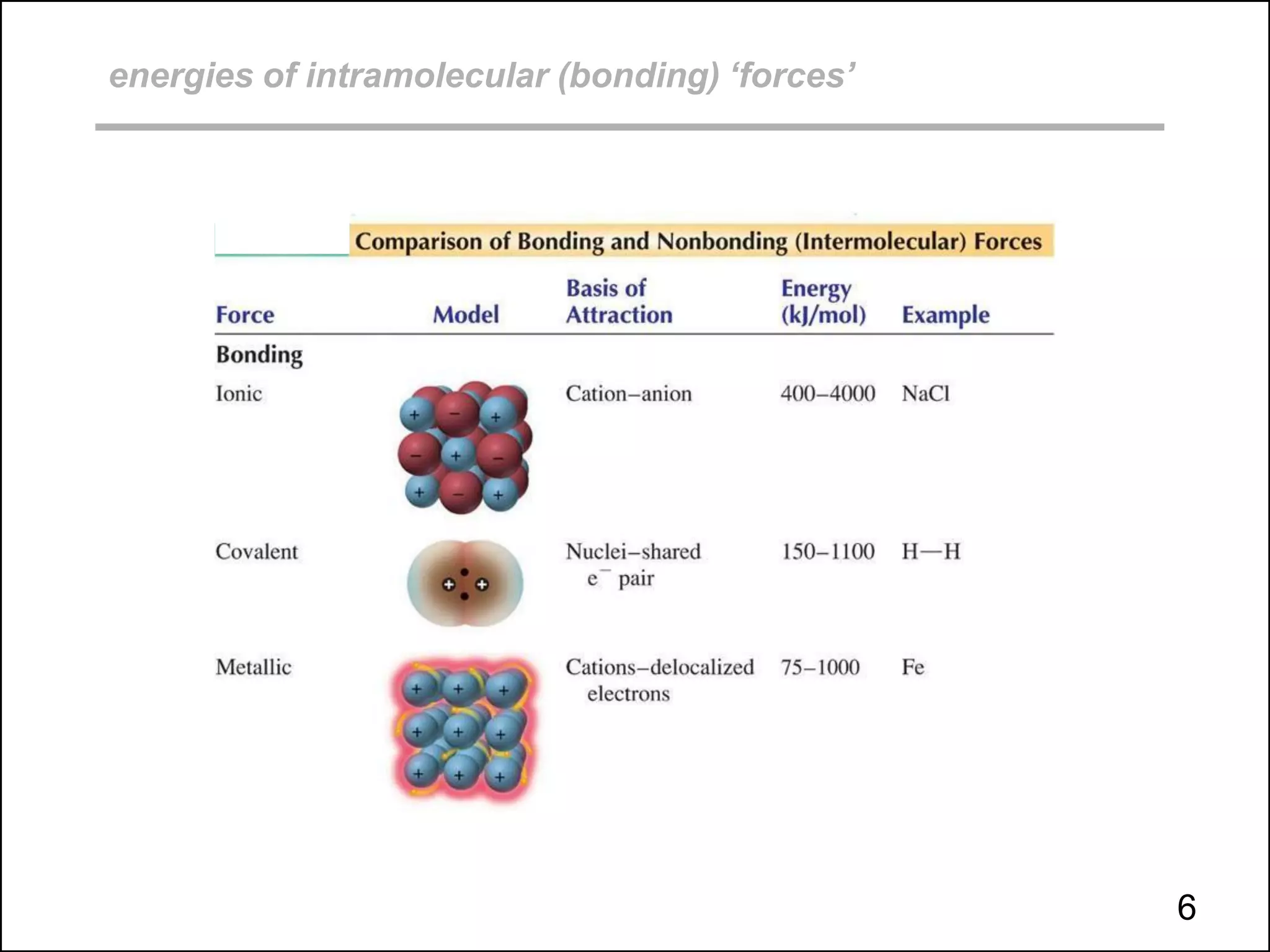
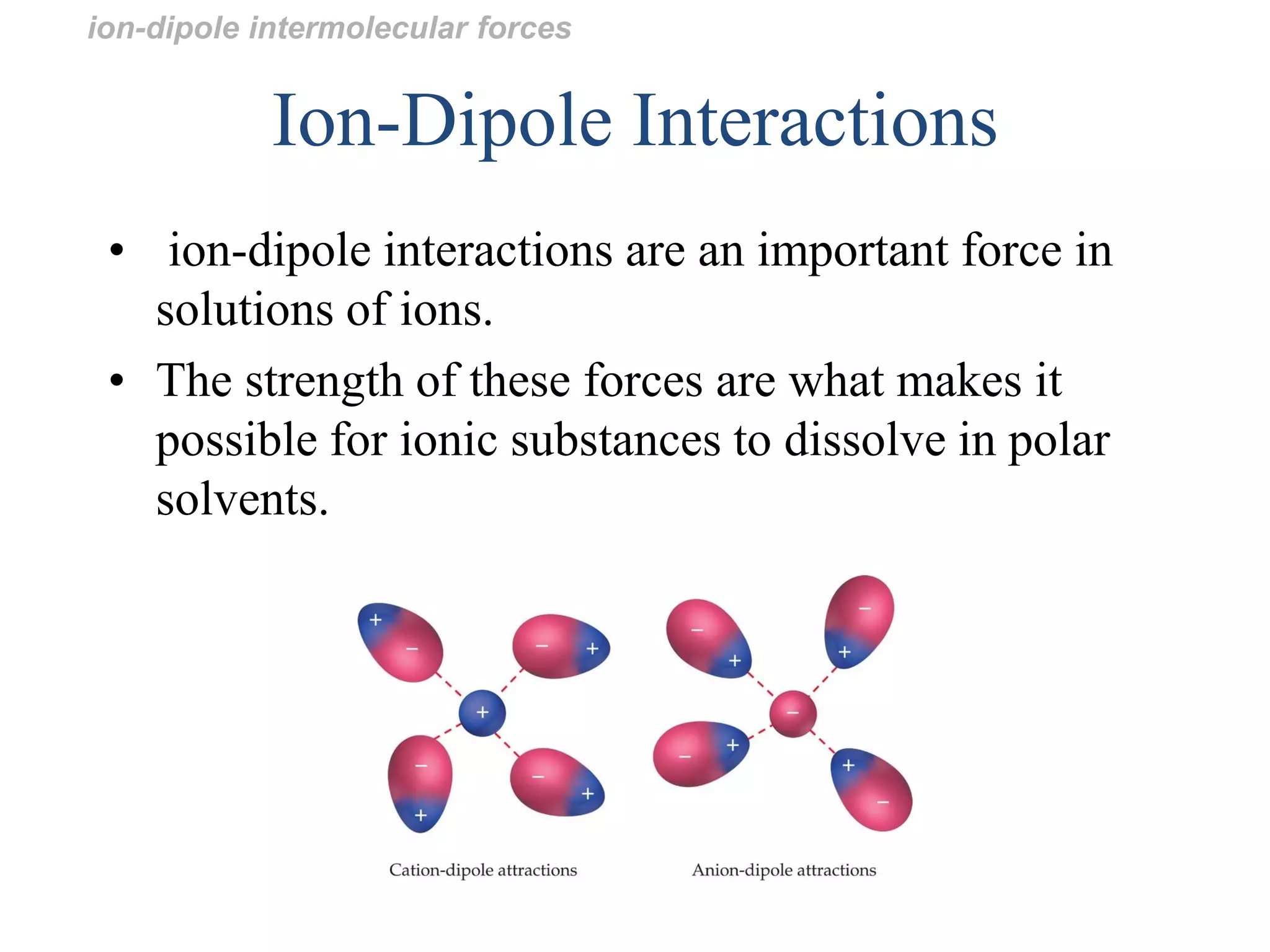
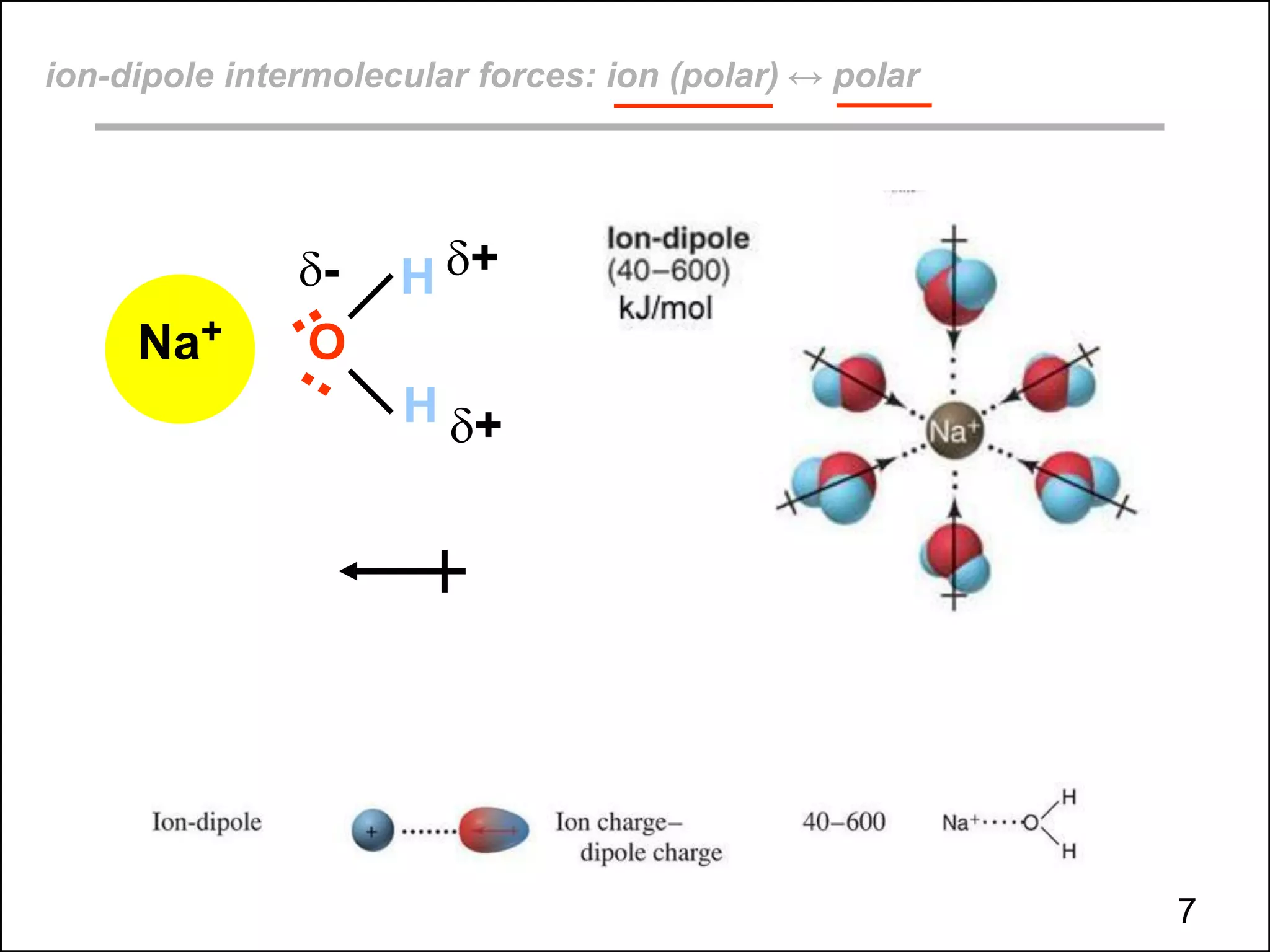
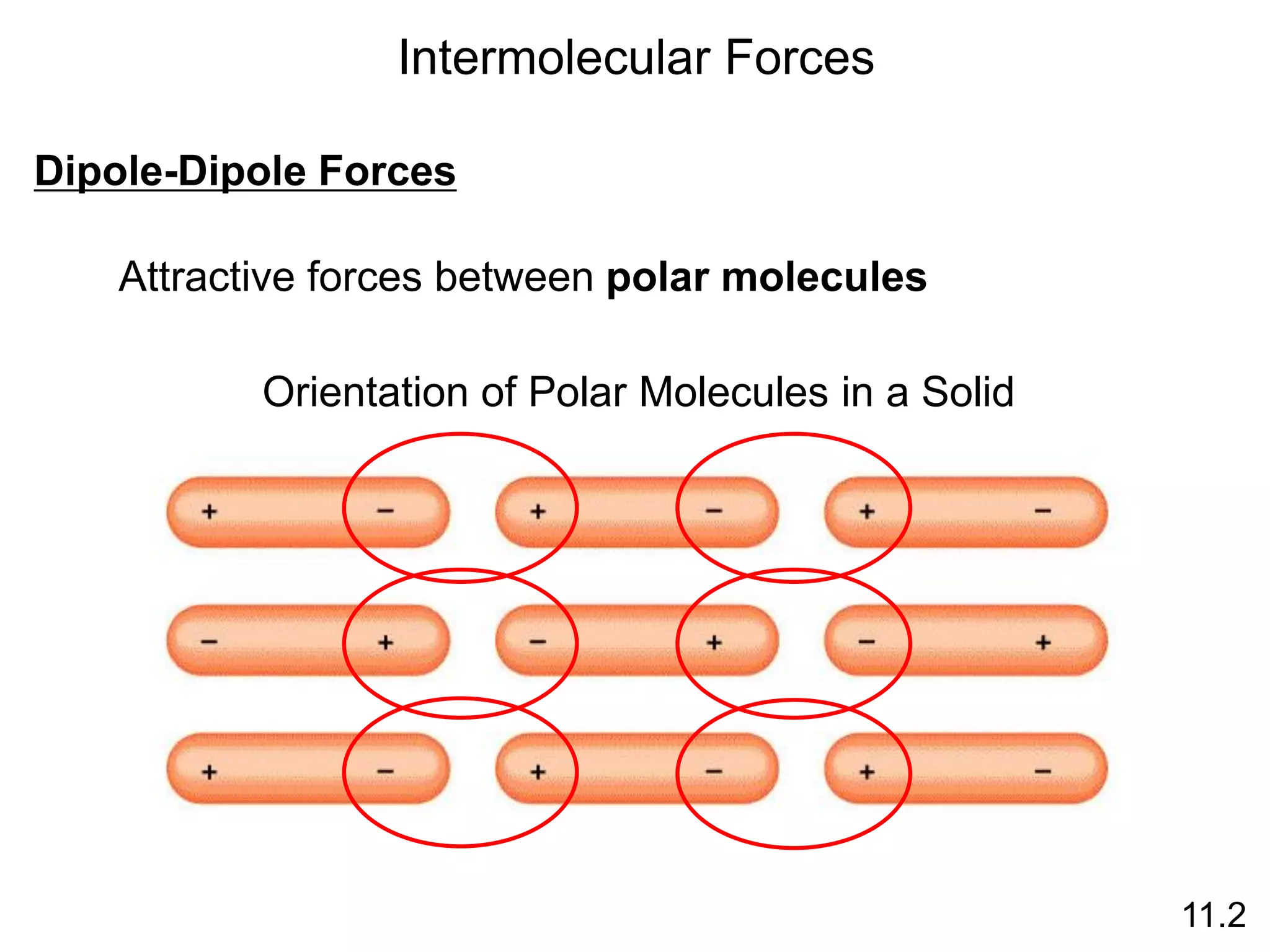
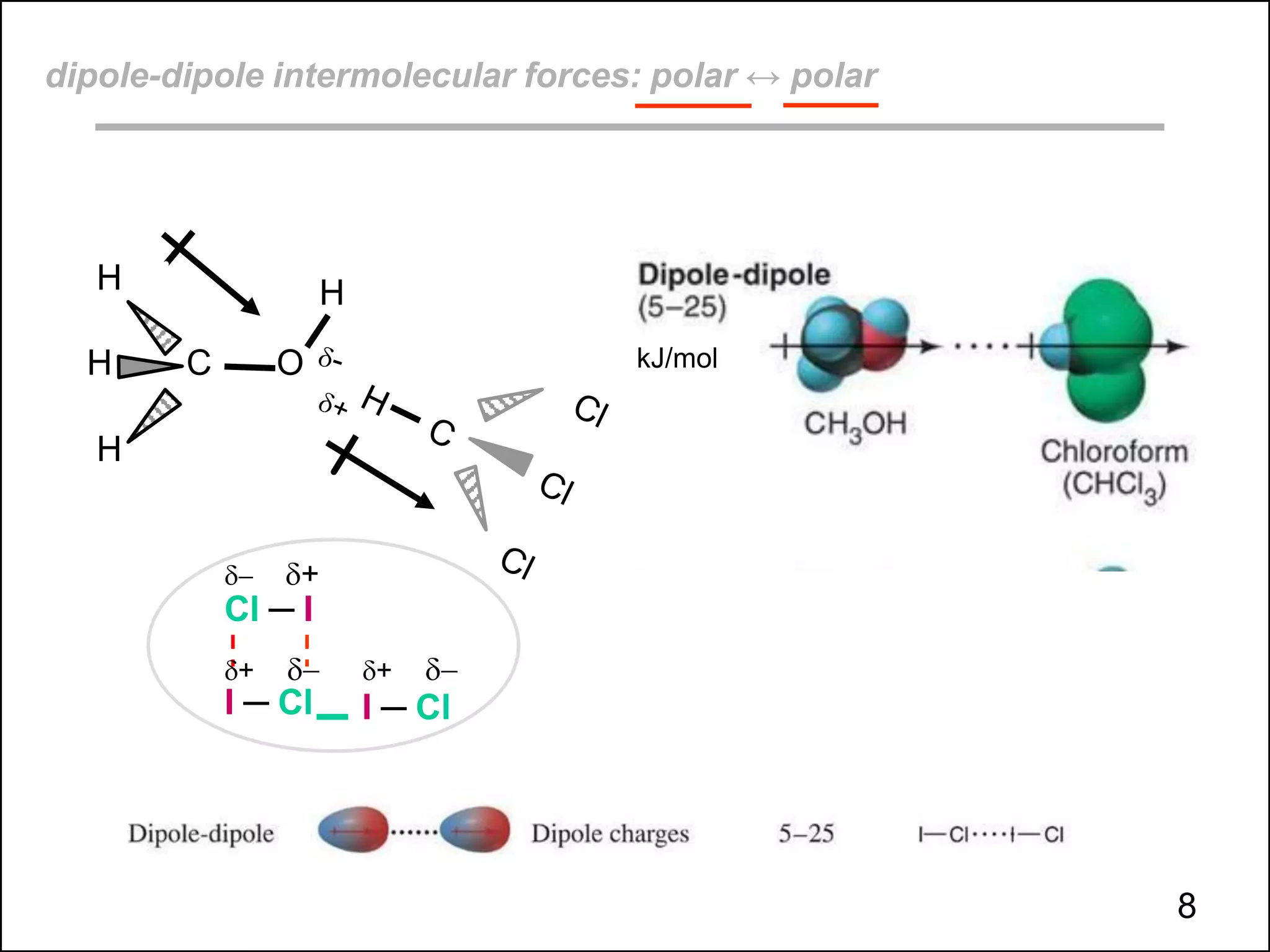
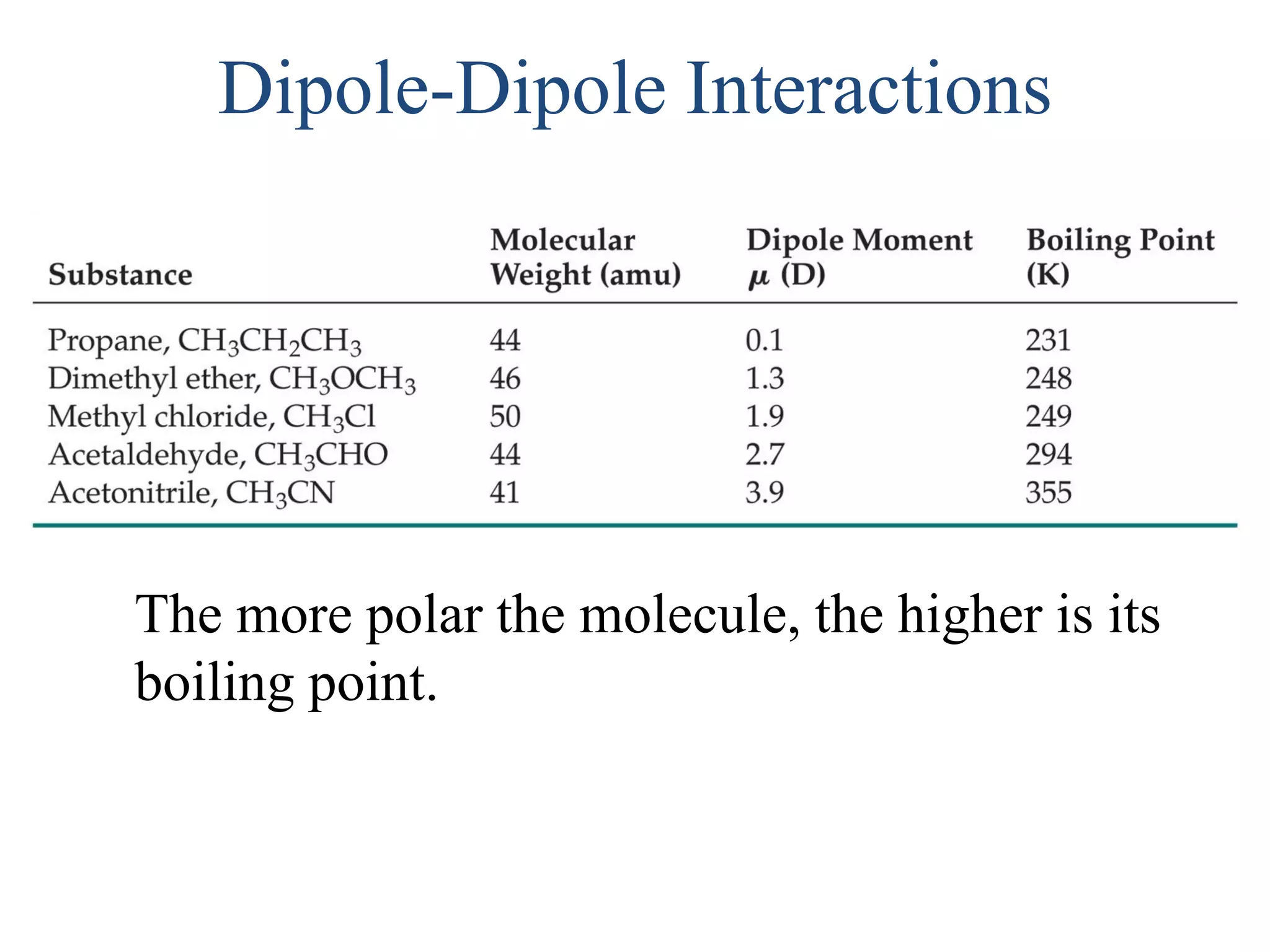
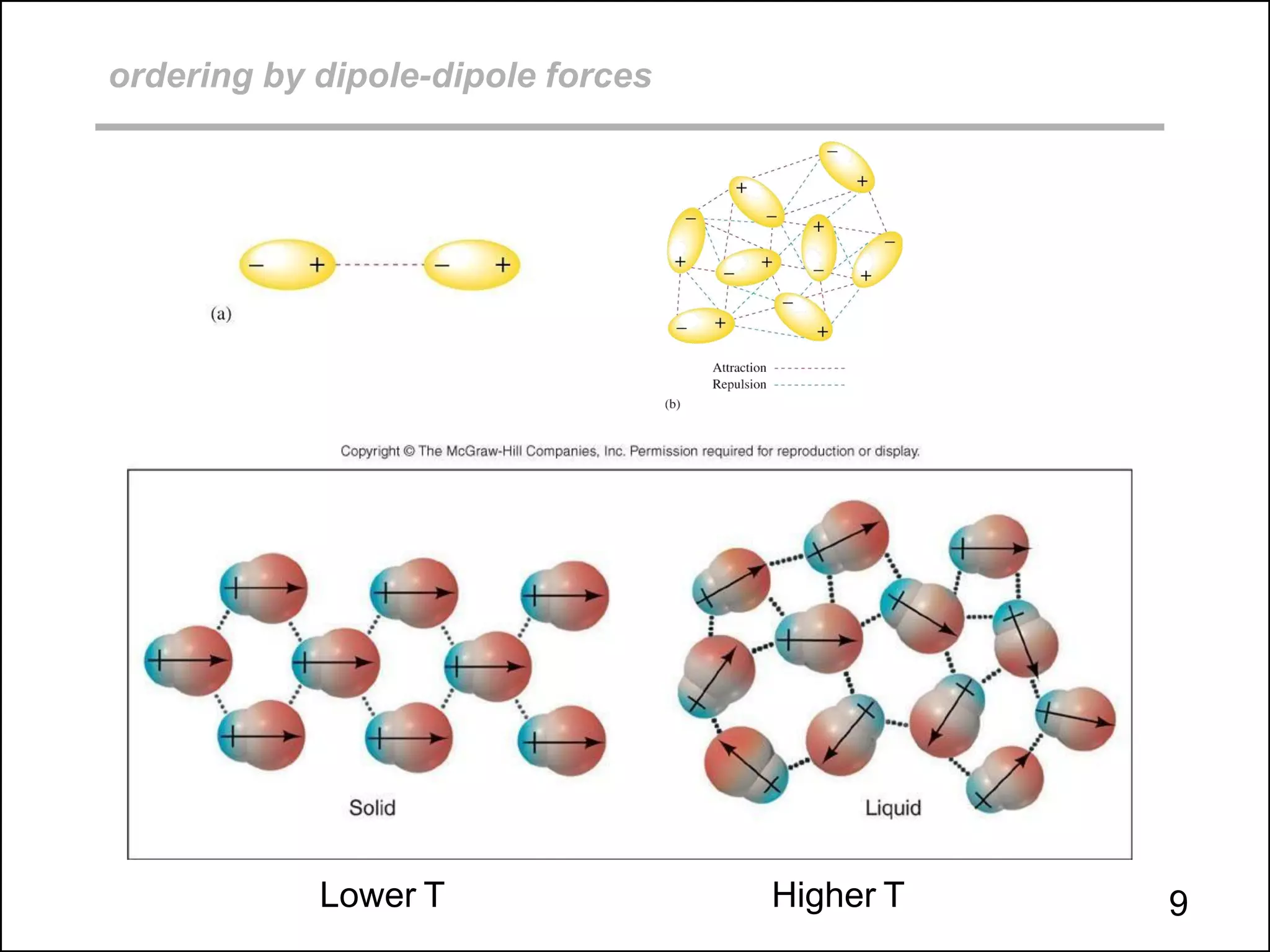
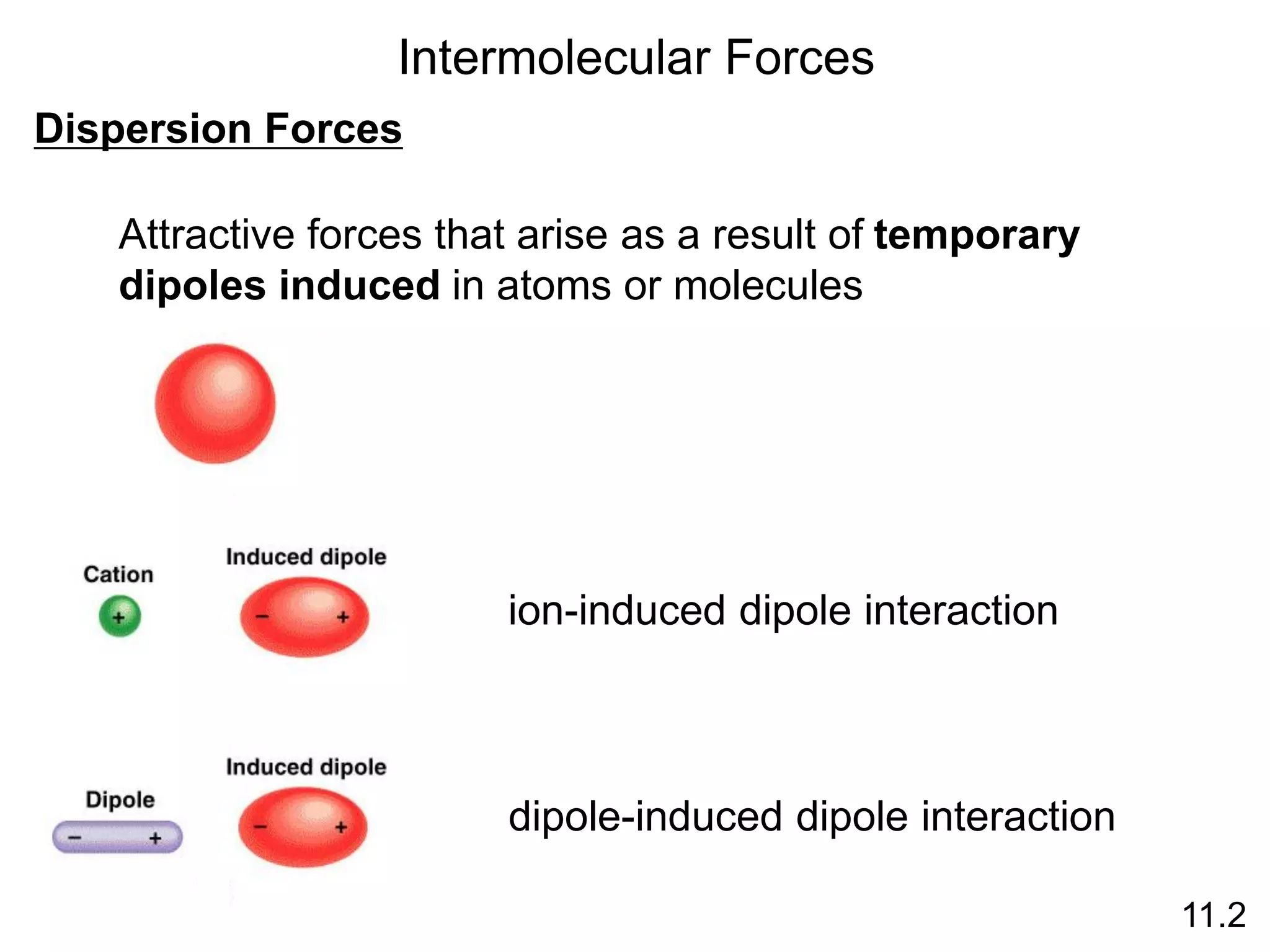
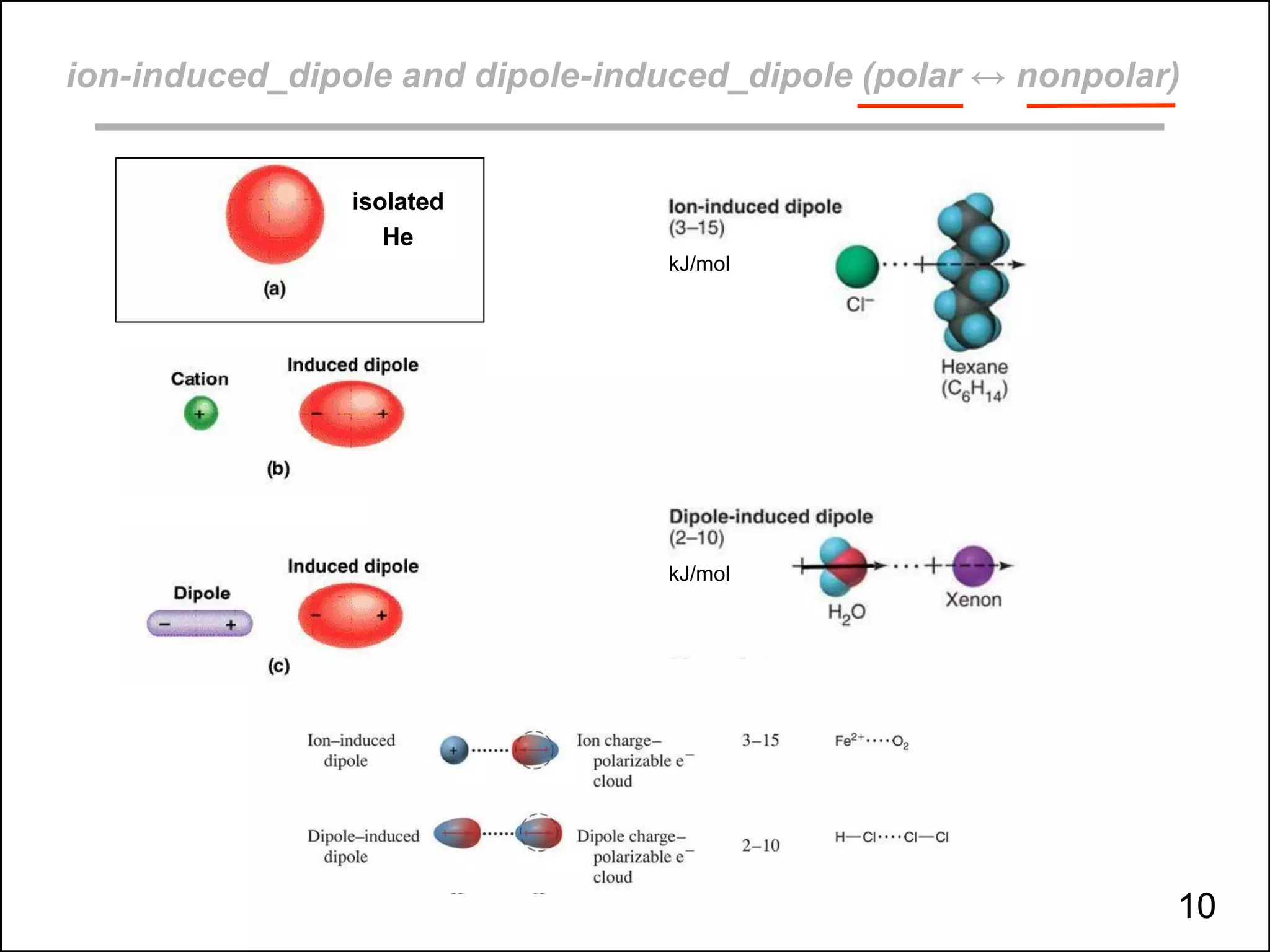
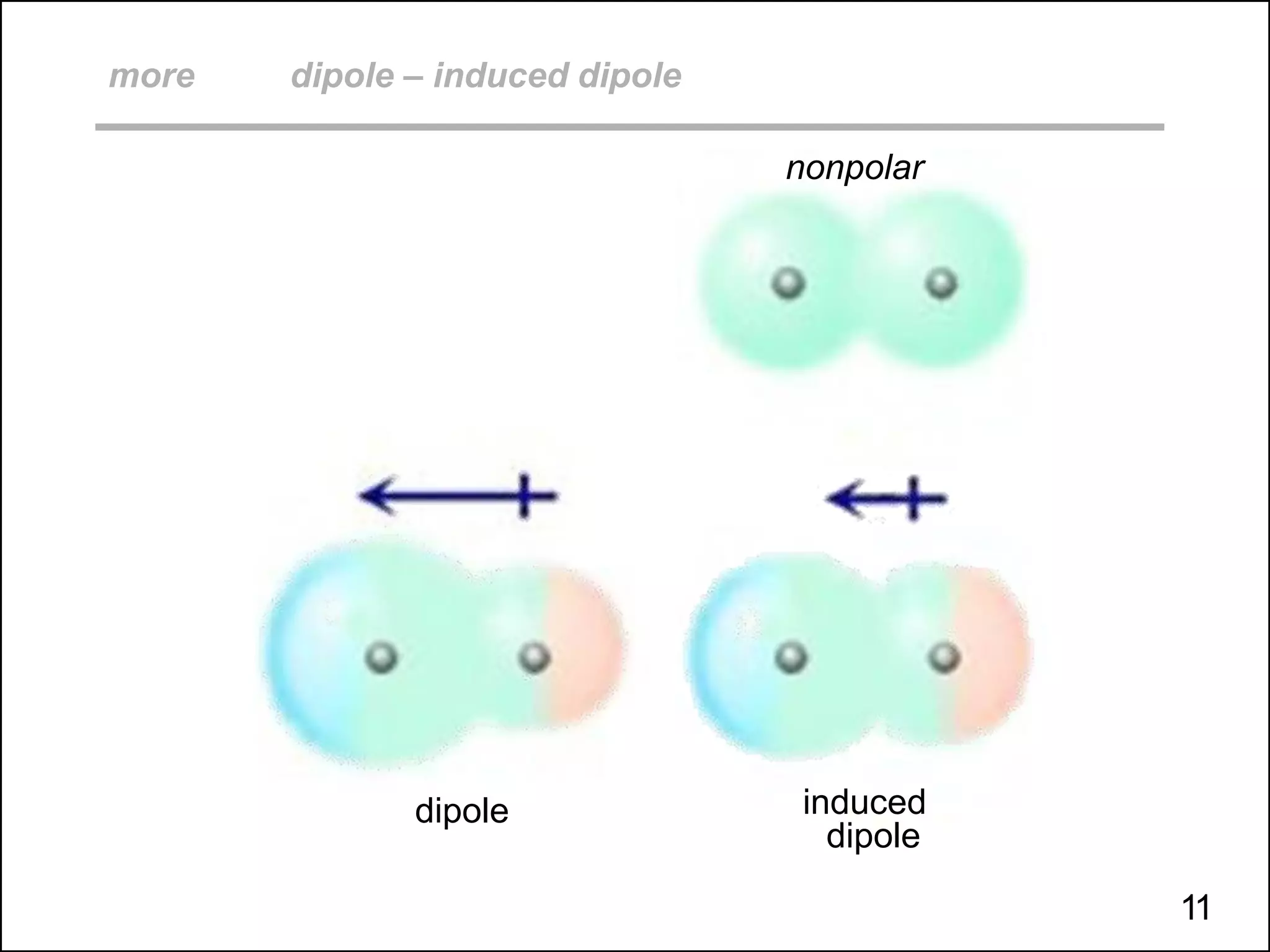
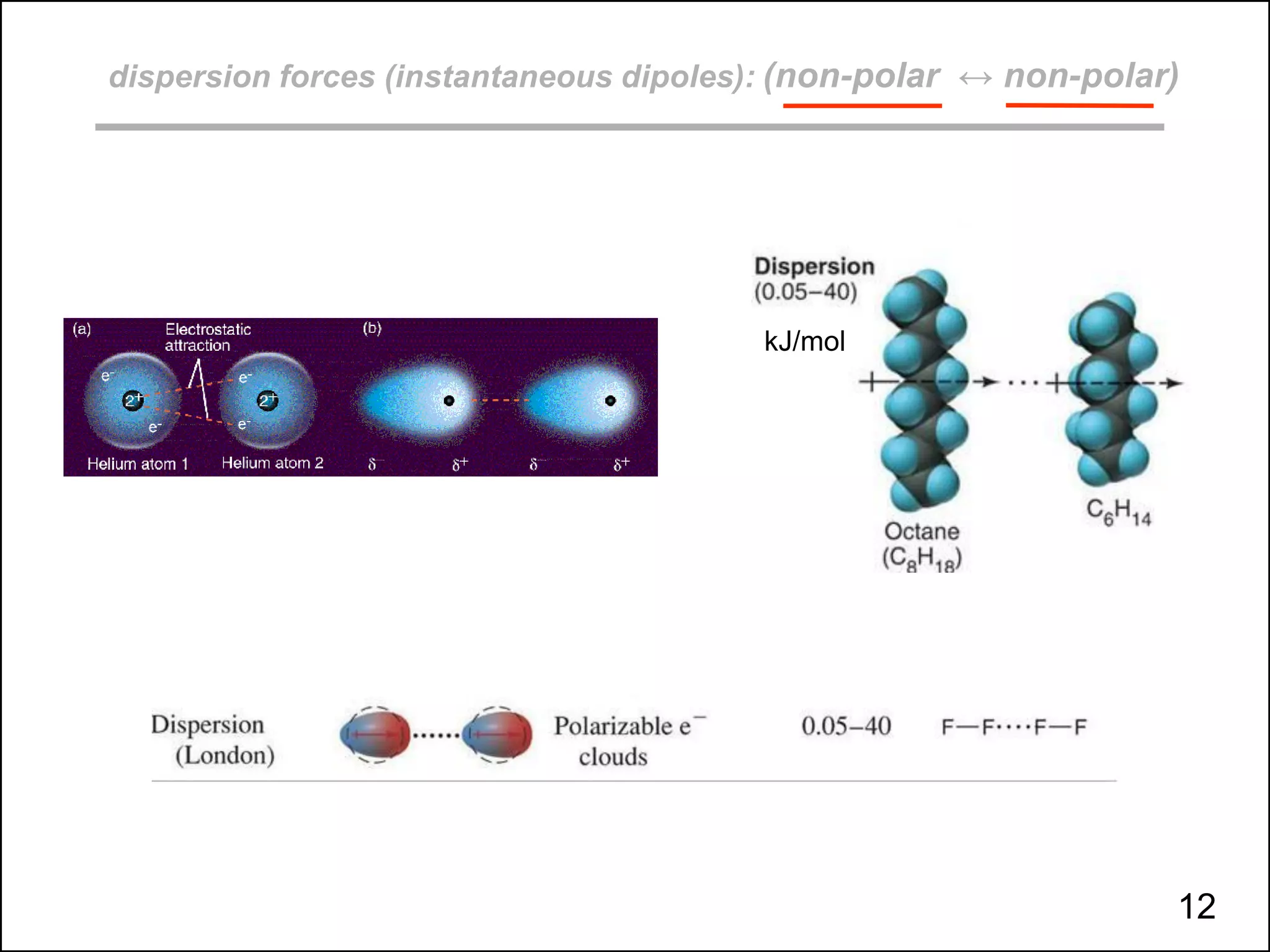
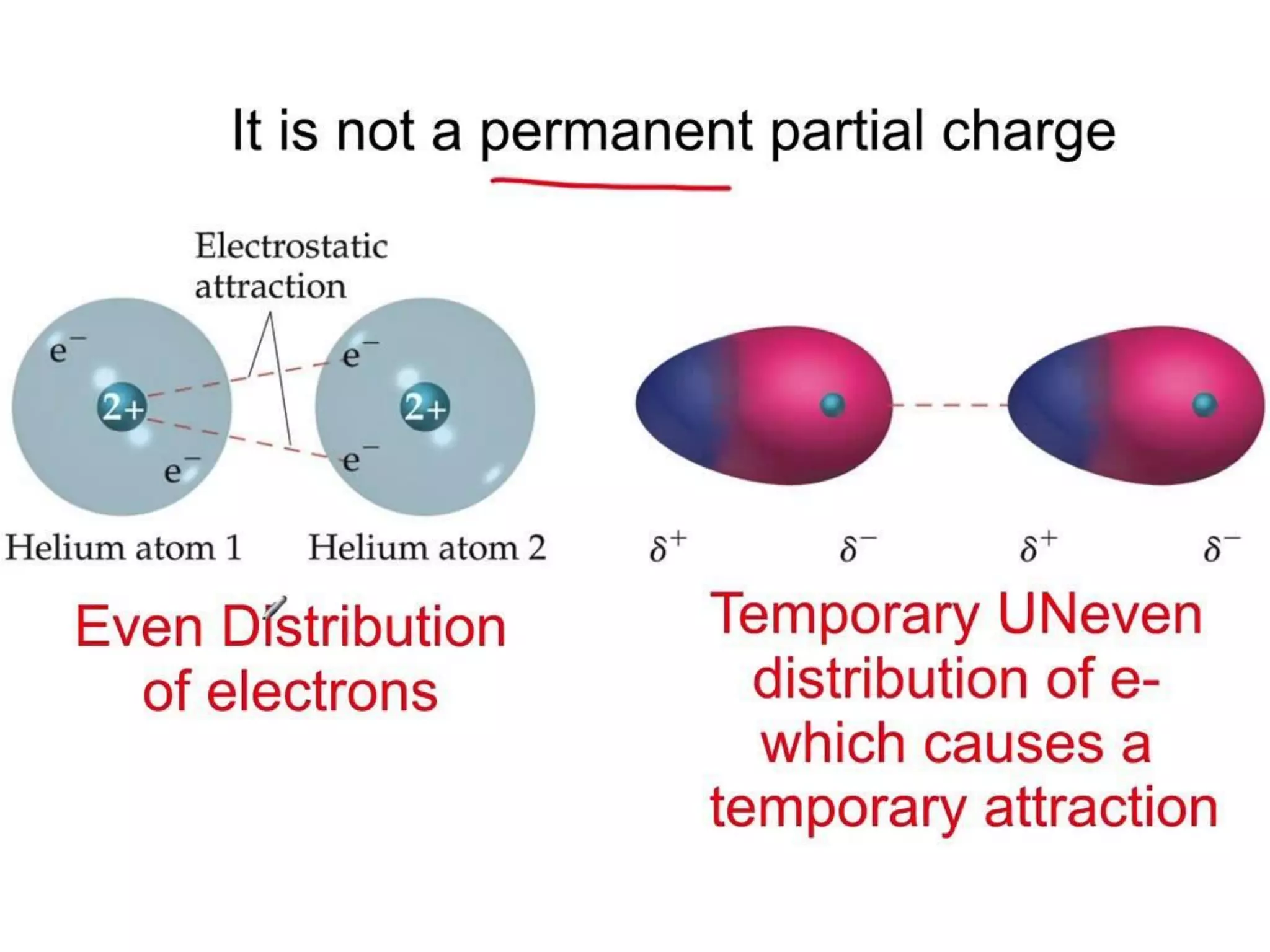
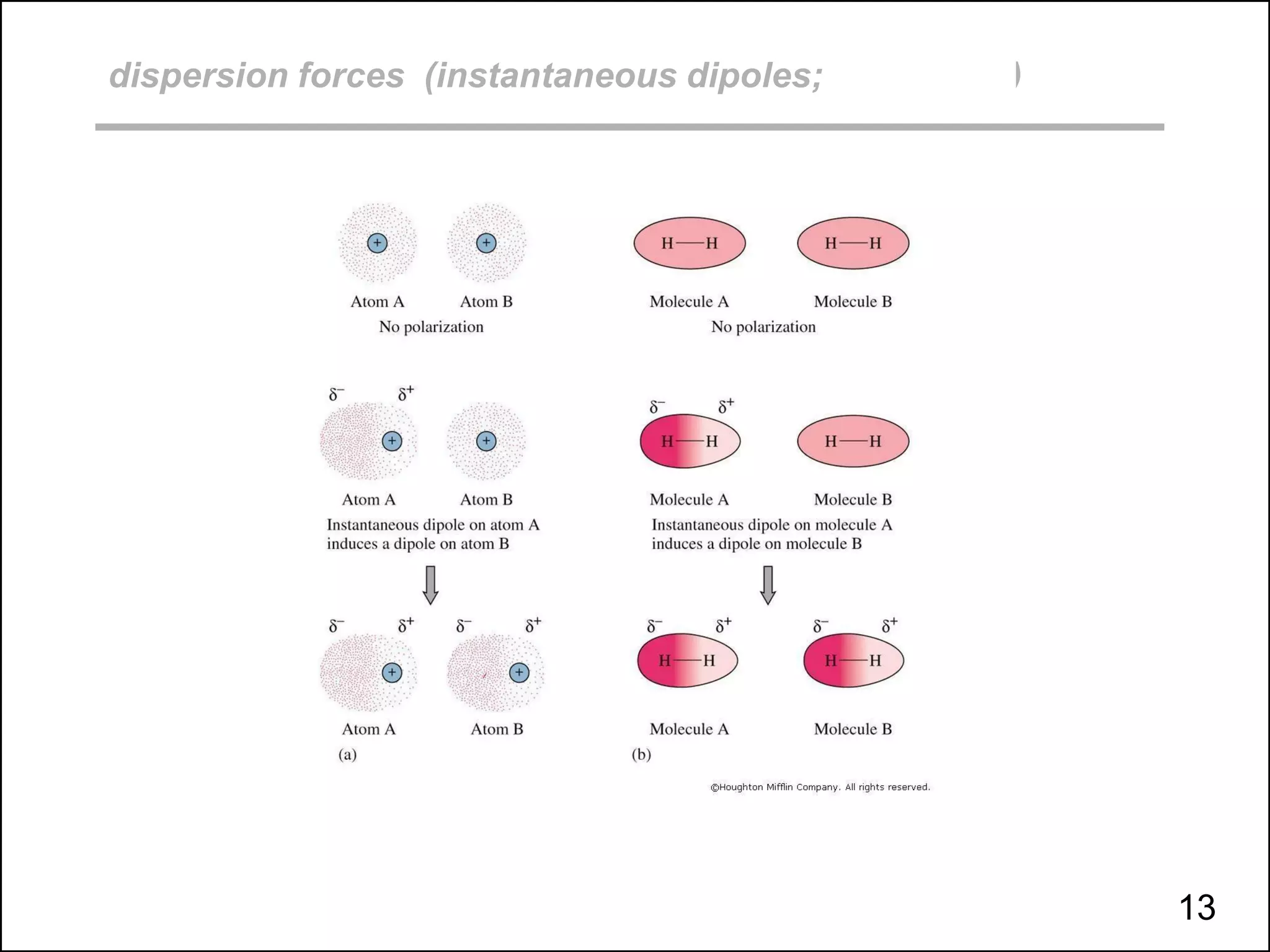
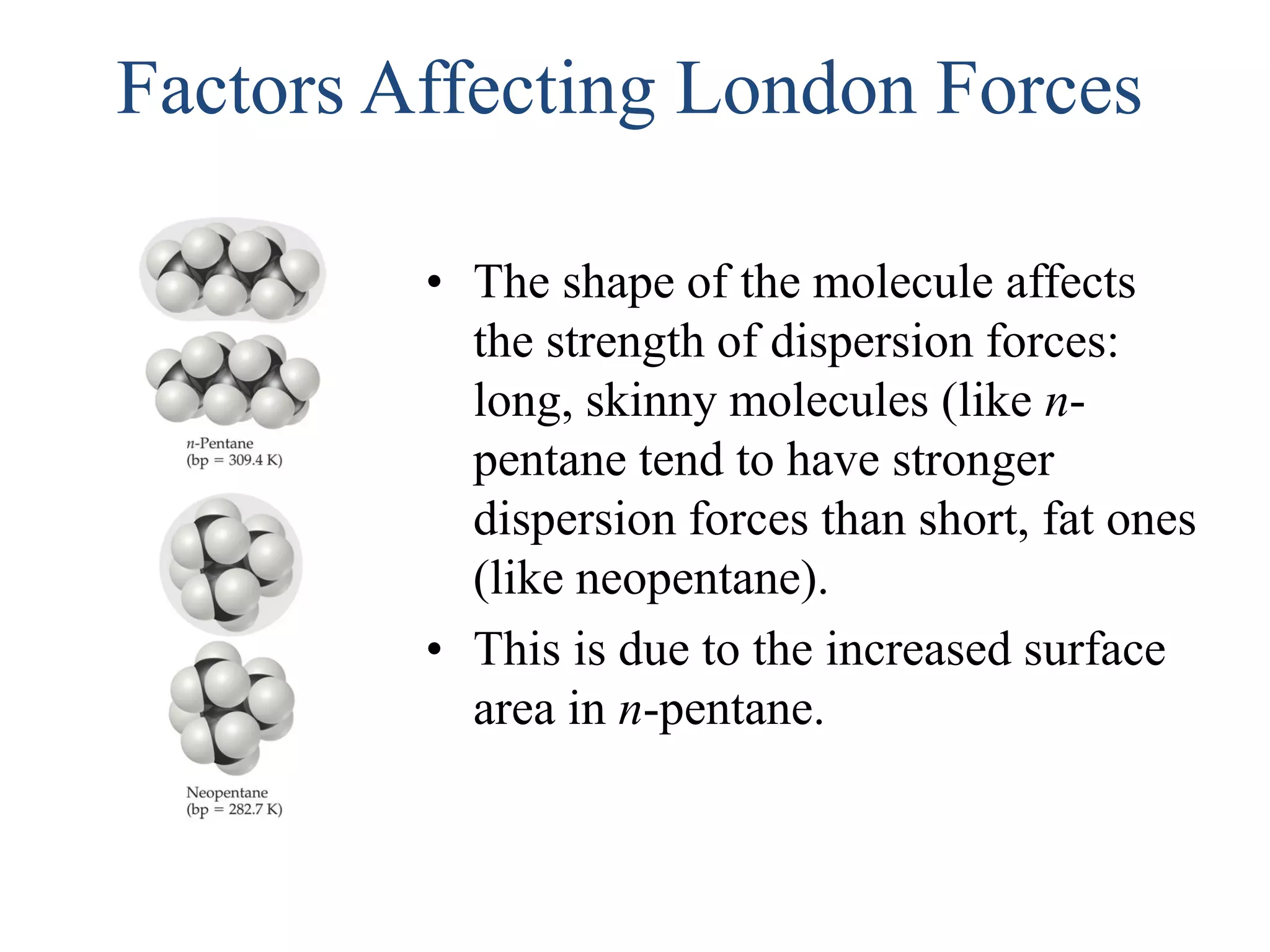
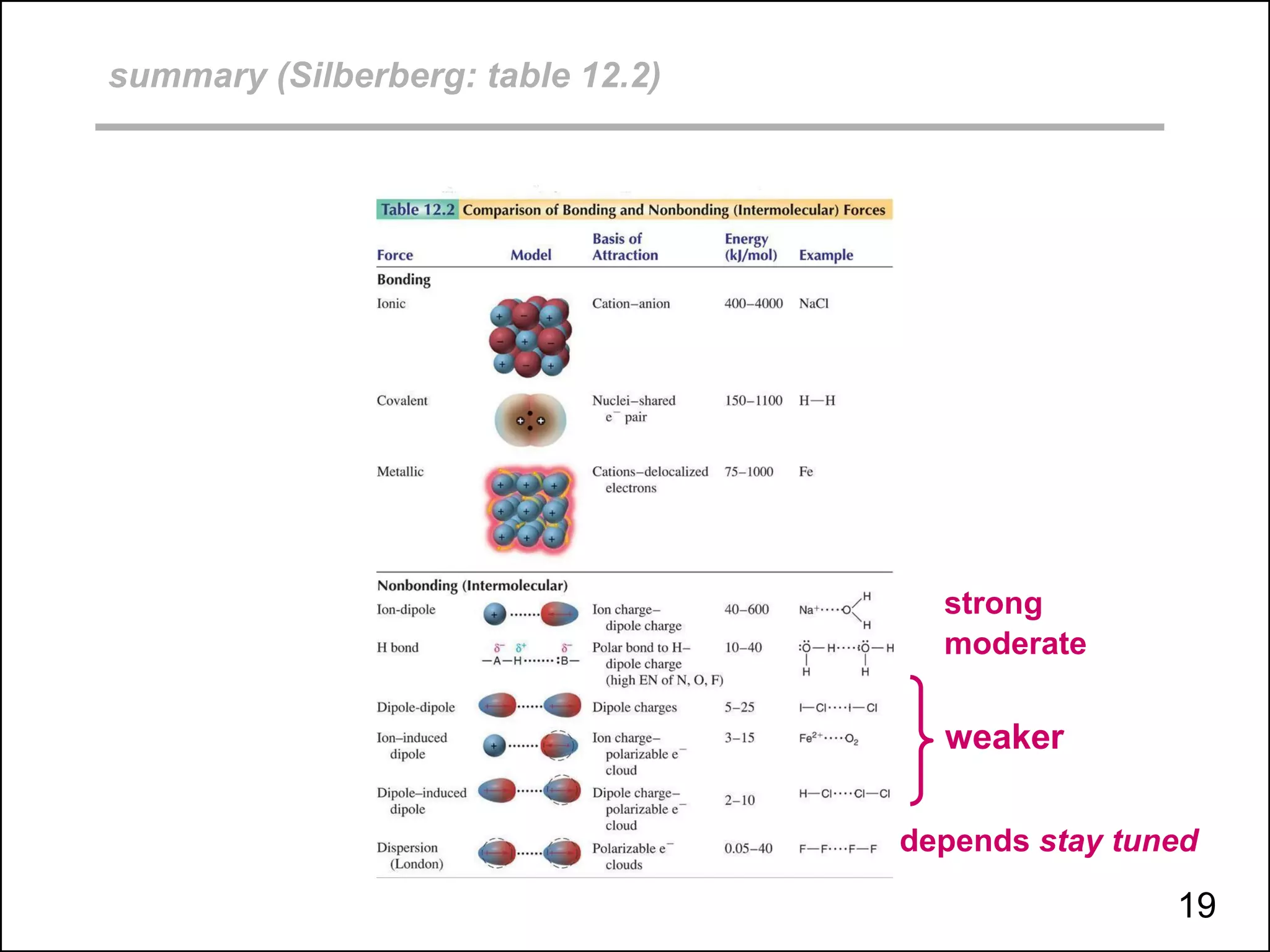
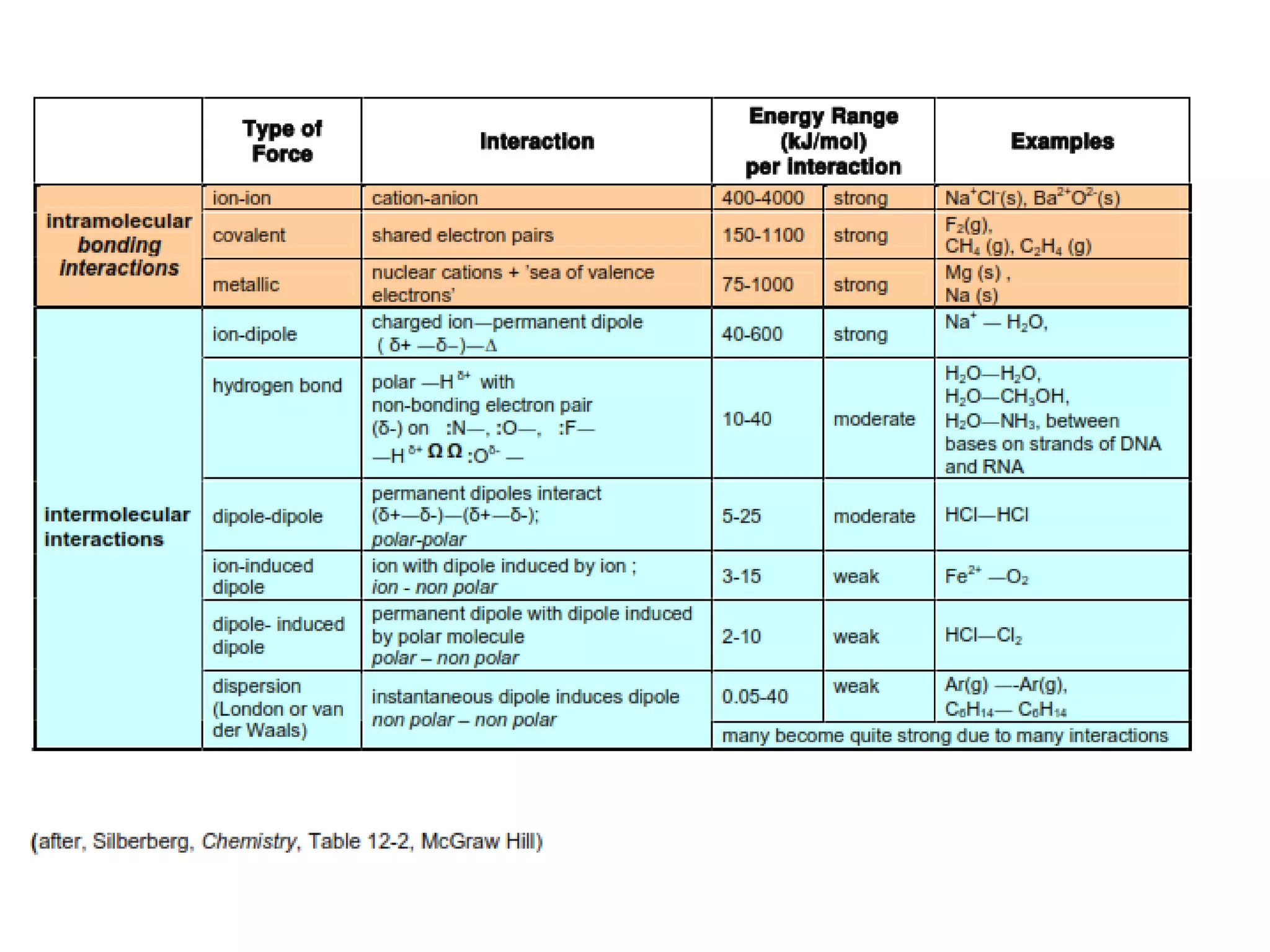
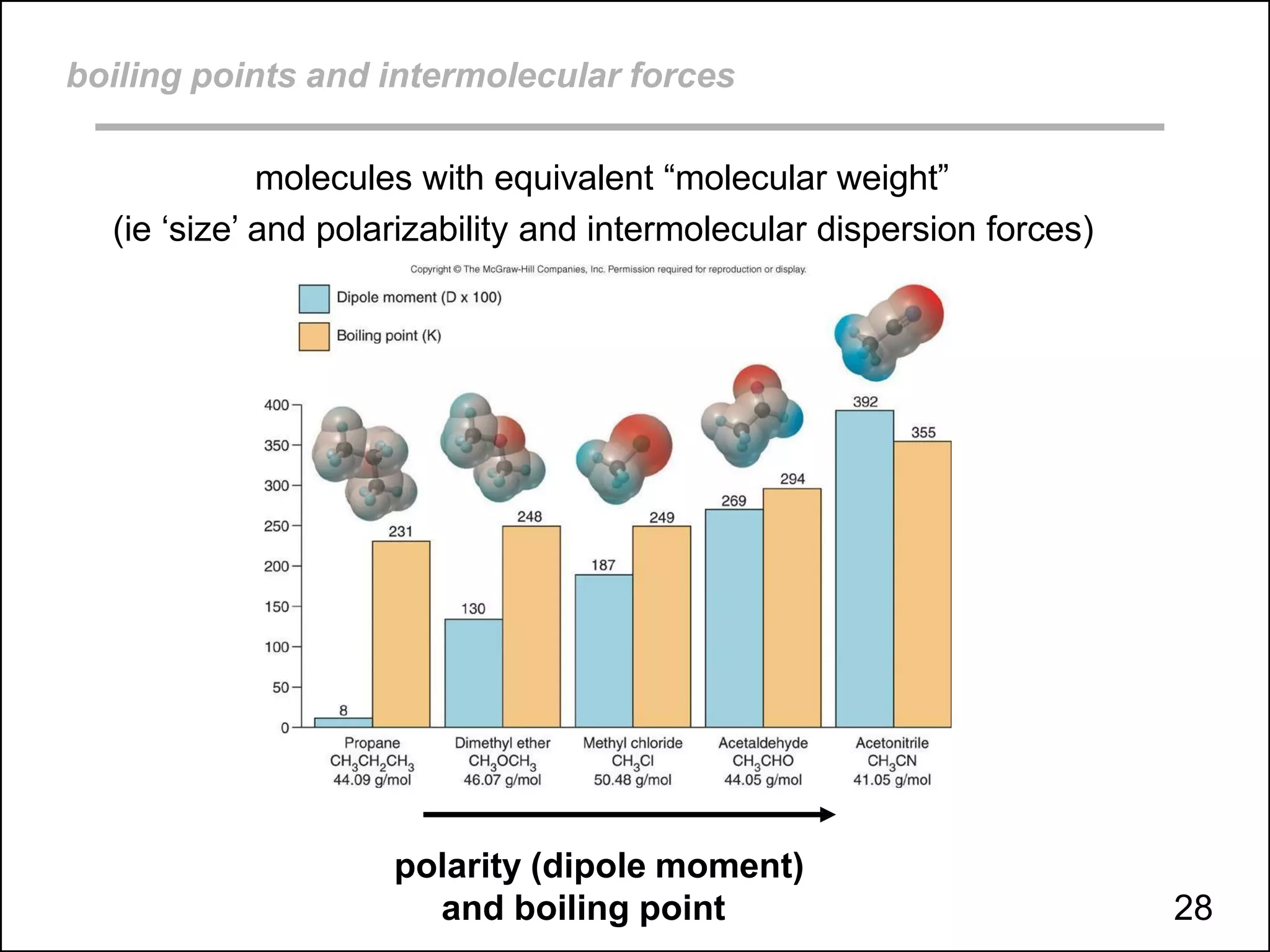
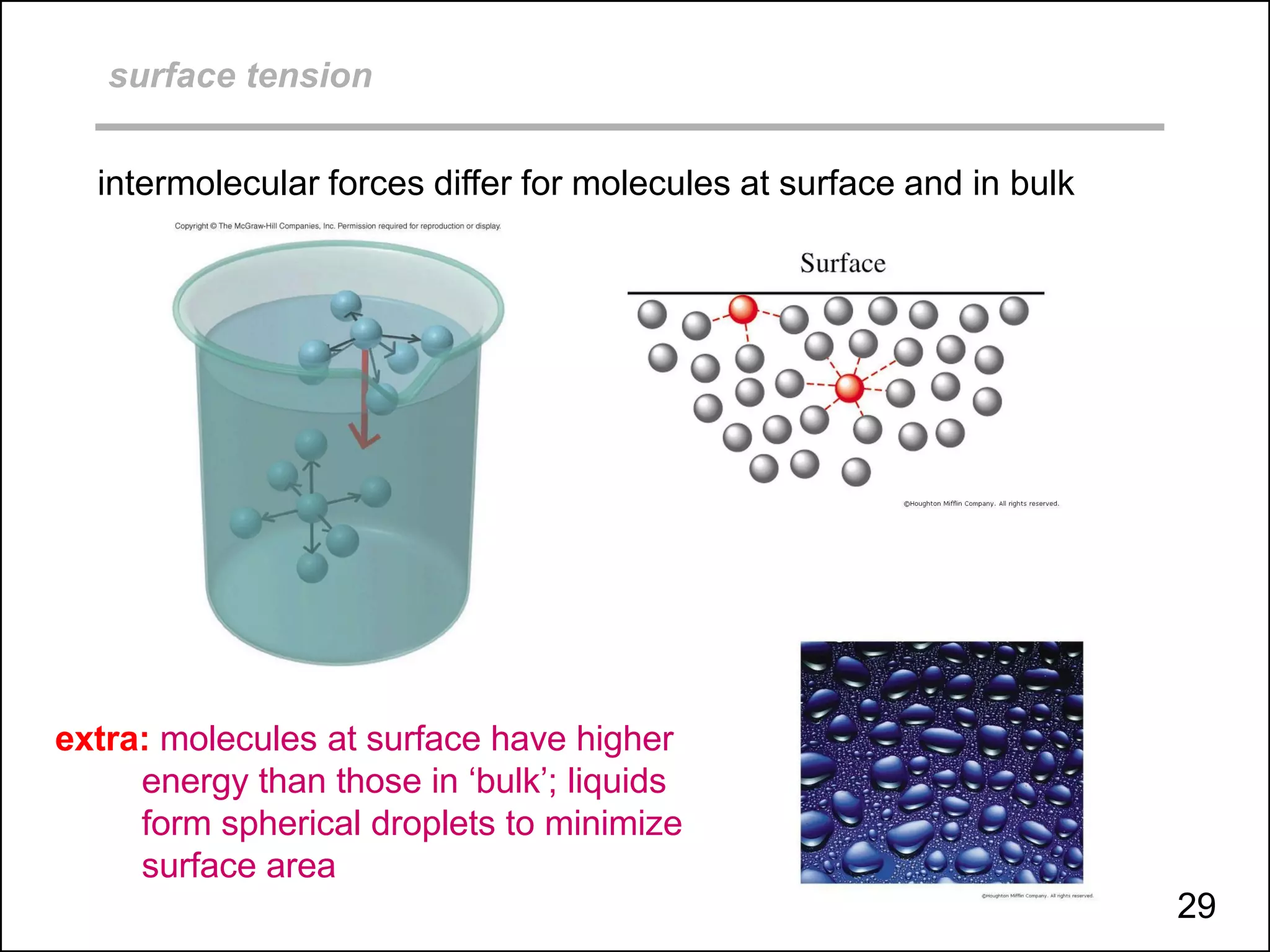
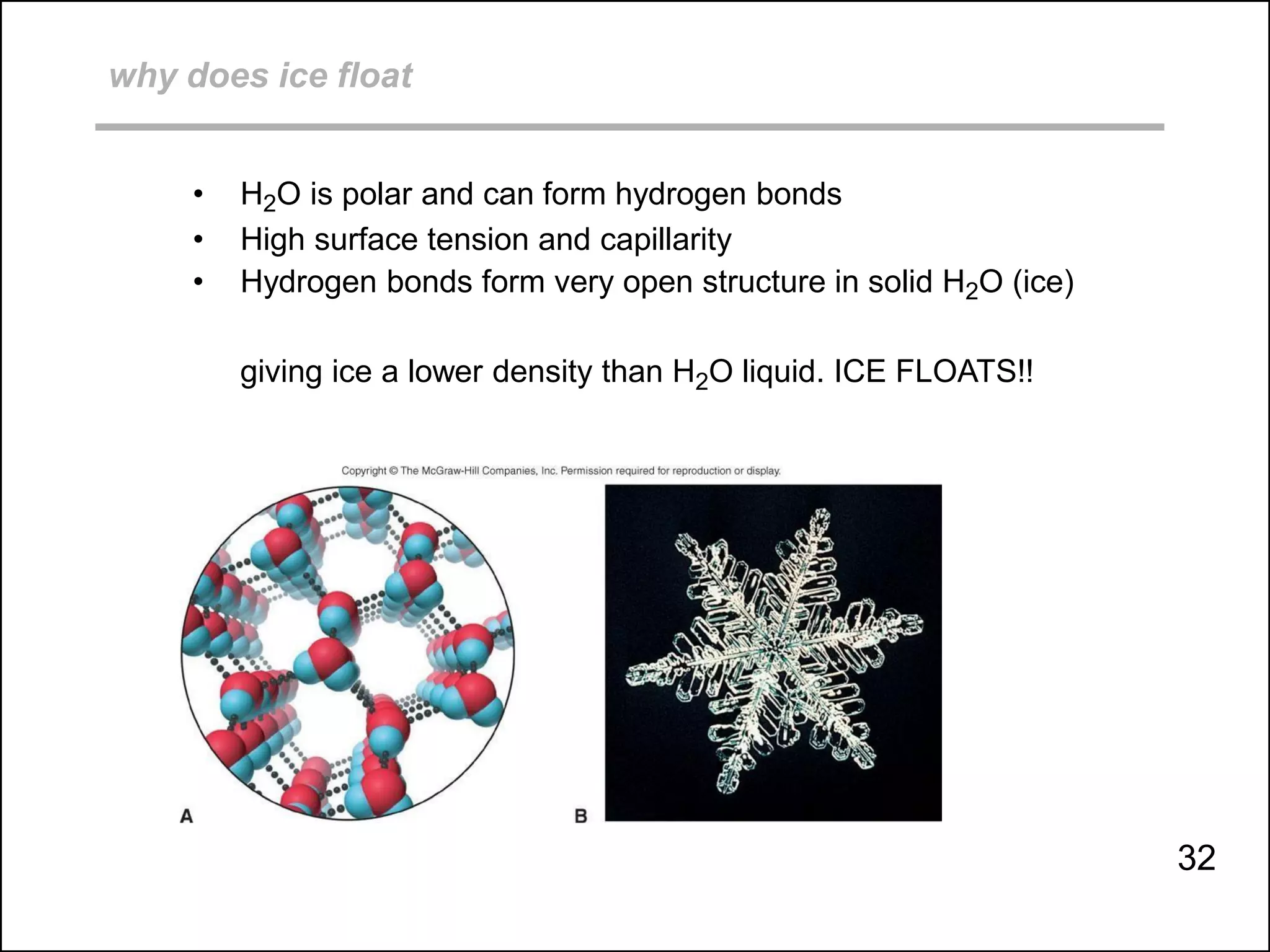
This document discusses different types of intermolecular and intramolecular forces. It begins by defining intramolecular forces as those within a molecule, such as covalent, ionic, and metallic bonds. Intermolecular forces are defined as those between different molecules, including ion-dipole, hydrogen bonding, dipole-dipole, and dispersion forces. The document then discusses how these different intermolecular forces relate to physical properties like boiling points, solubility, surface tension, and phase changes. It emphasizes that stronger intermolecular forces lead to higher boiling points, freezing points, and surface tension as well as differences in solubility between polar and nonpolar substances.


![increased polarizability
increased freezing point
boiling point in Kº
23
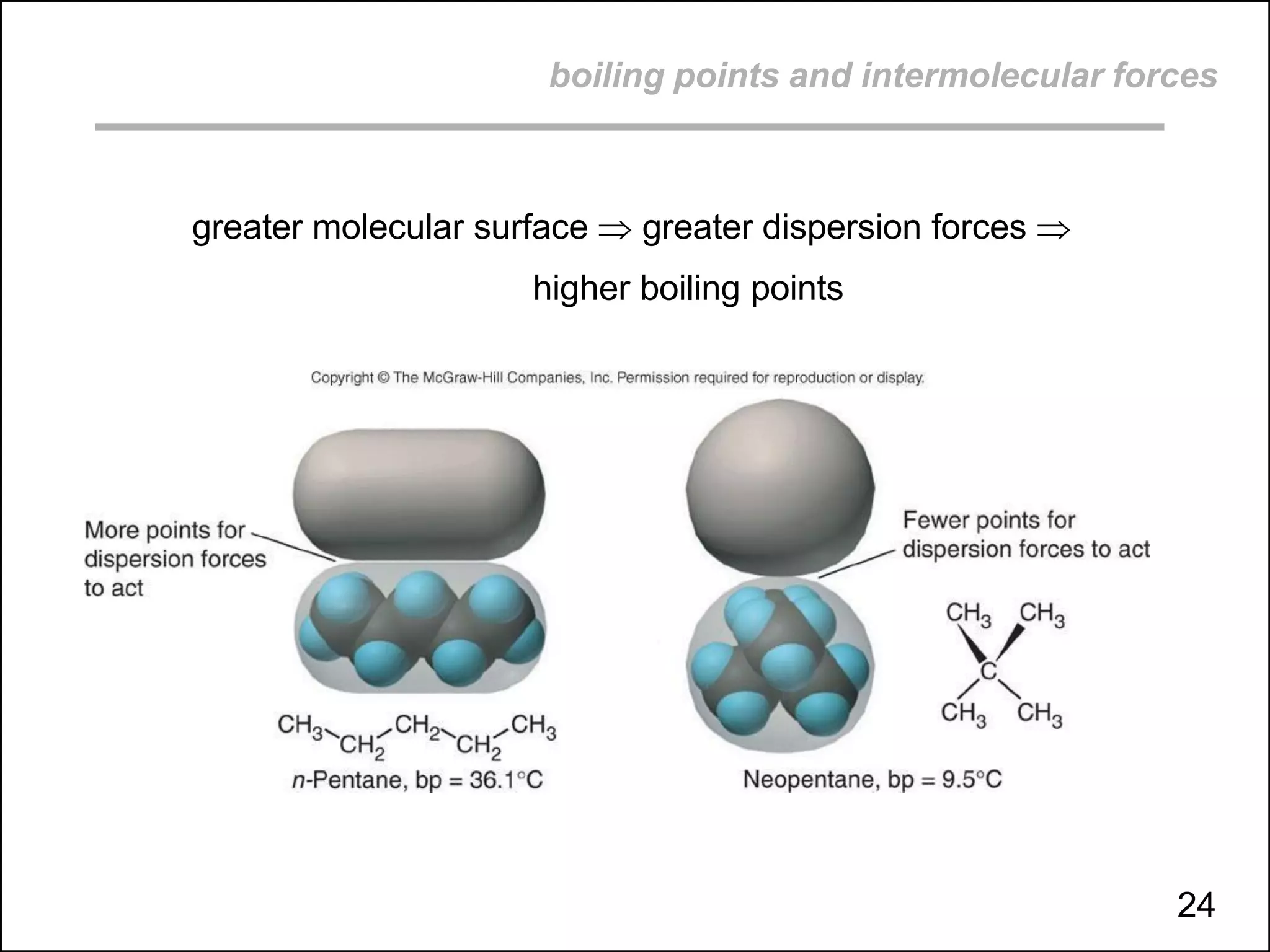
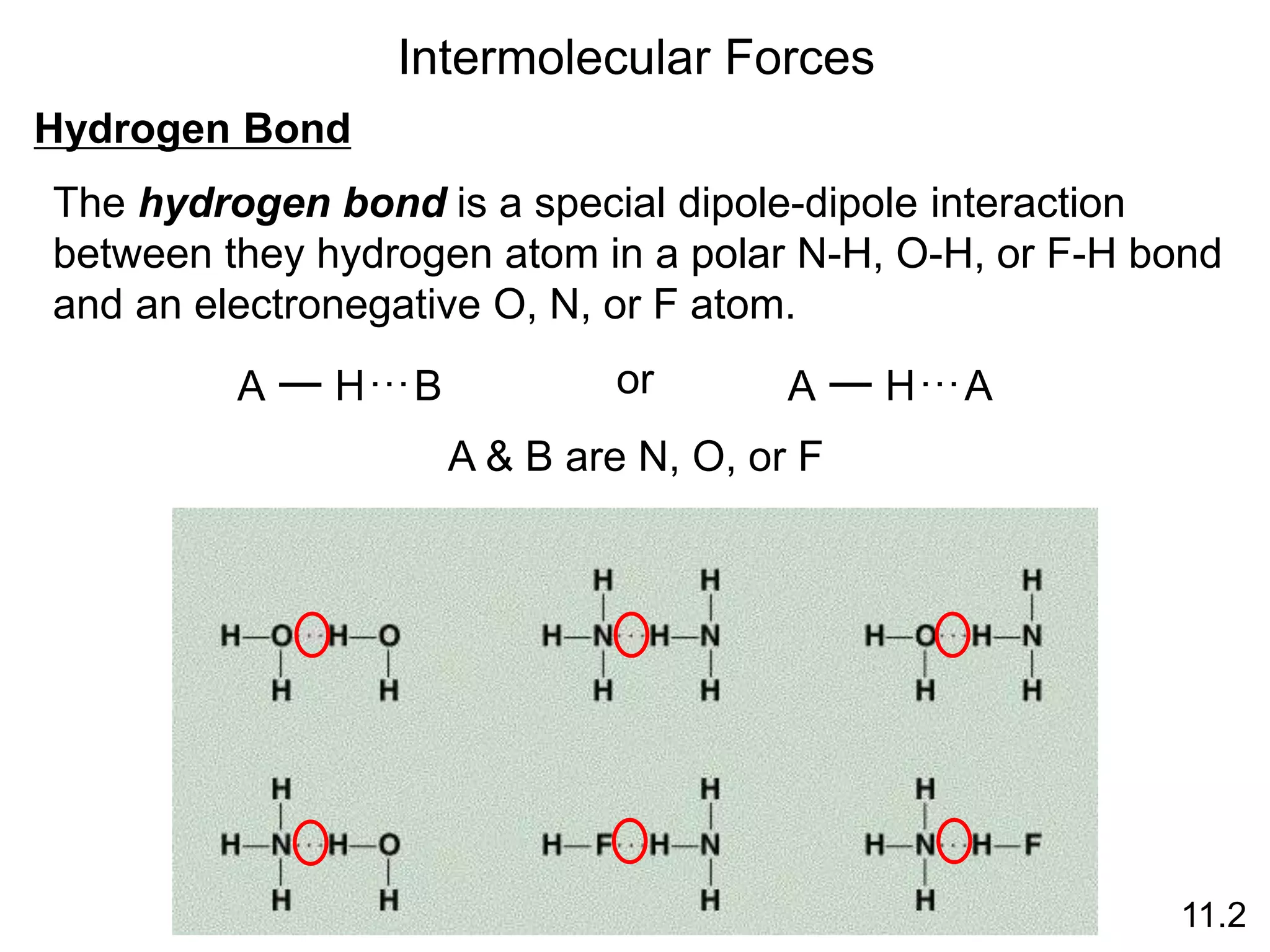
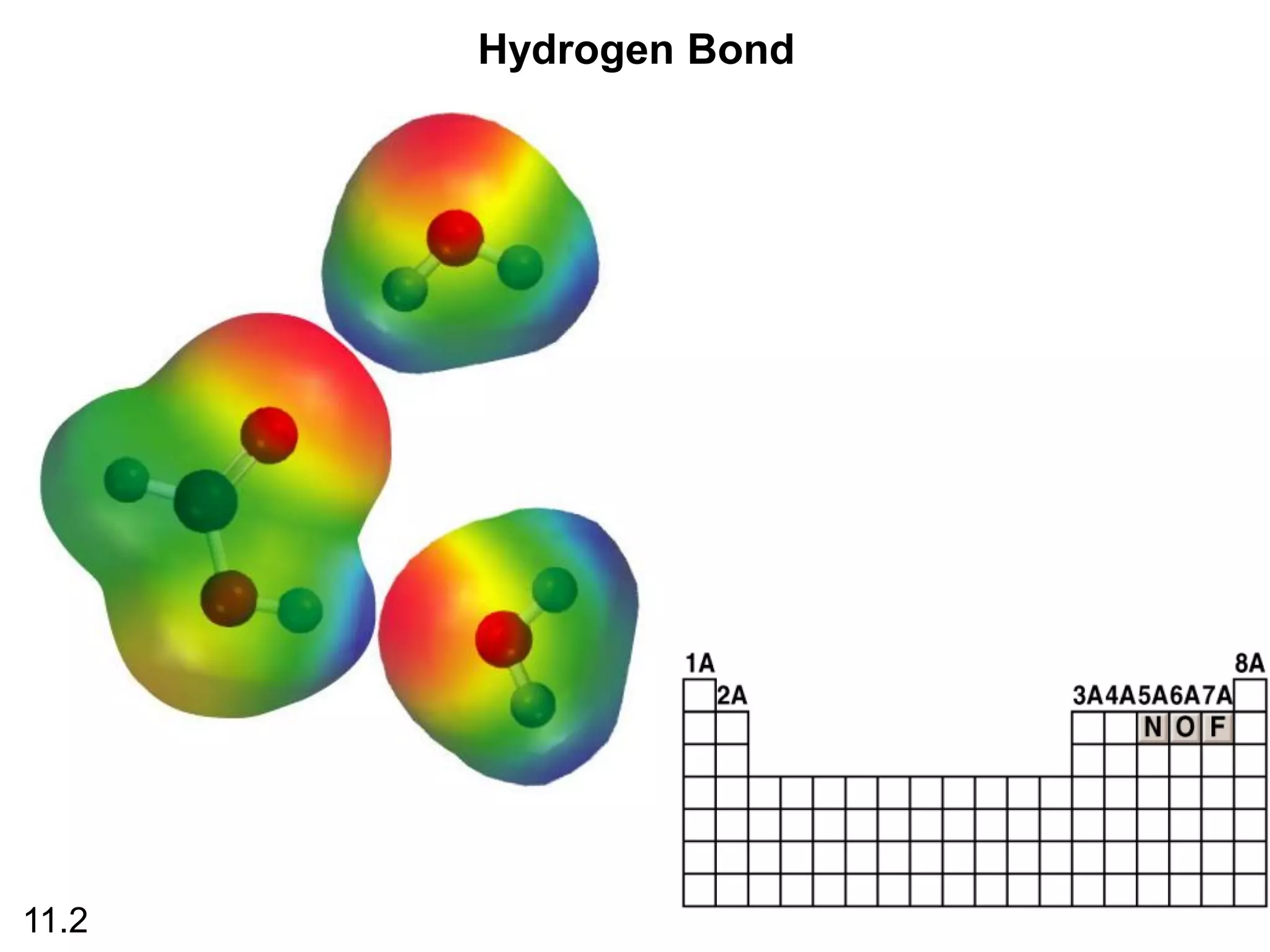
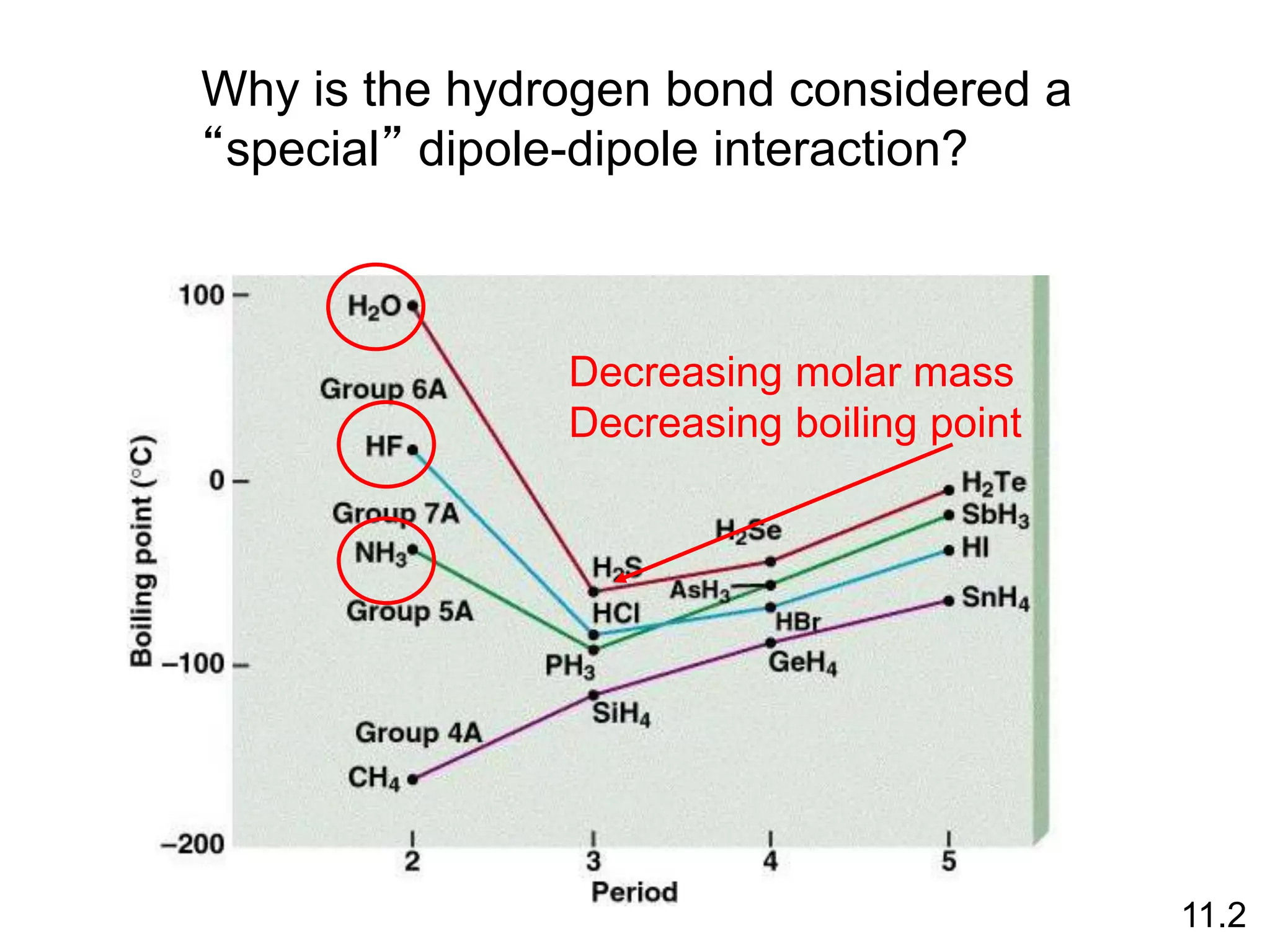
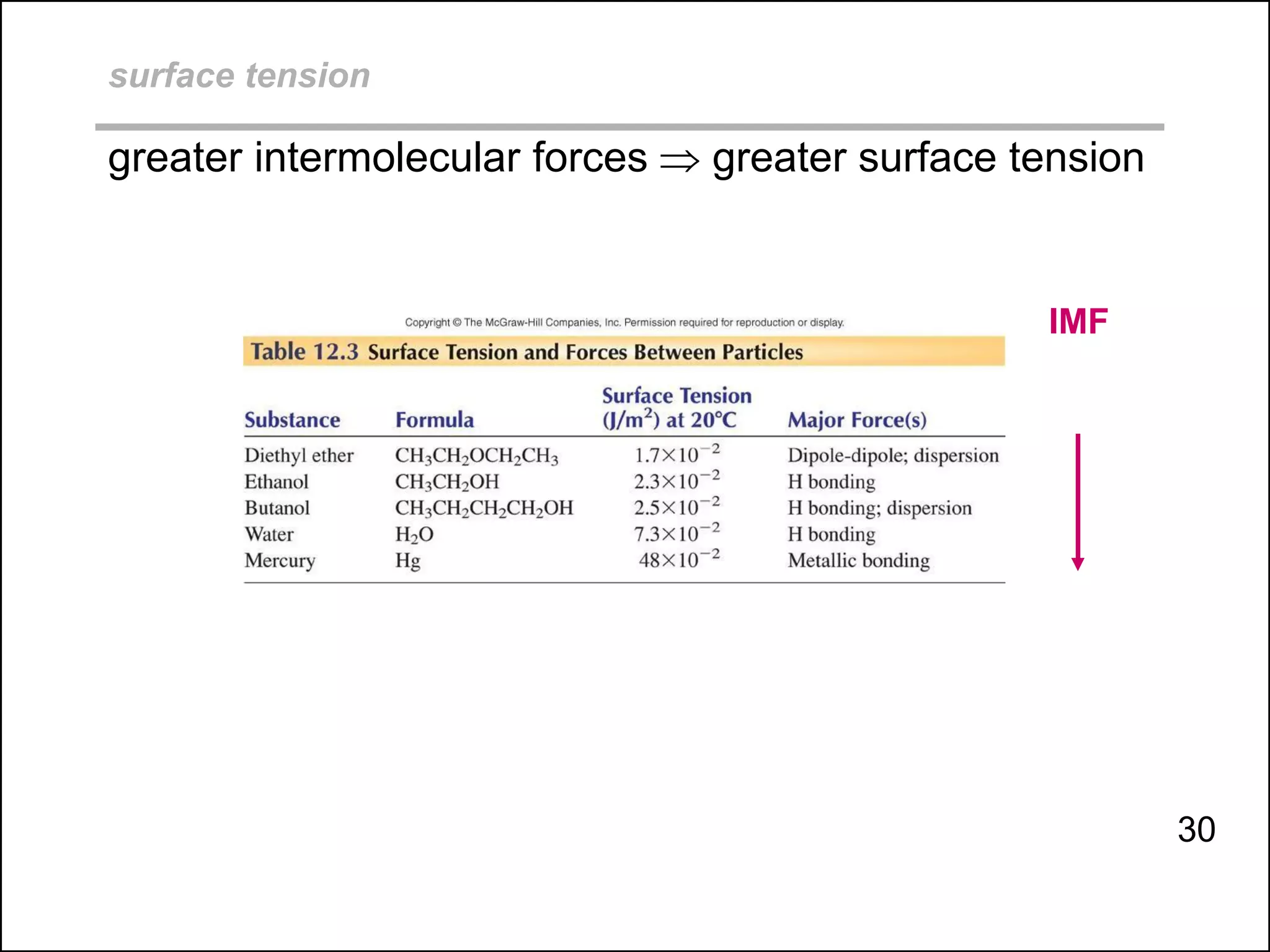
boiling points, melting points, vapor pressure and intermolecular forces
(nonpolar compounds)
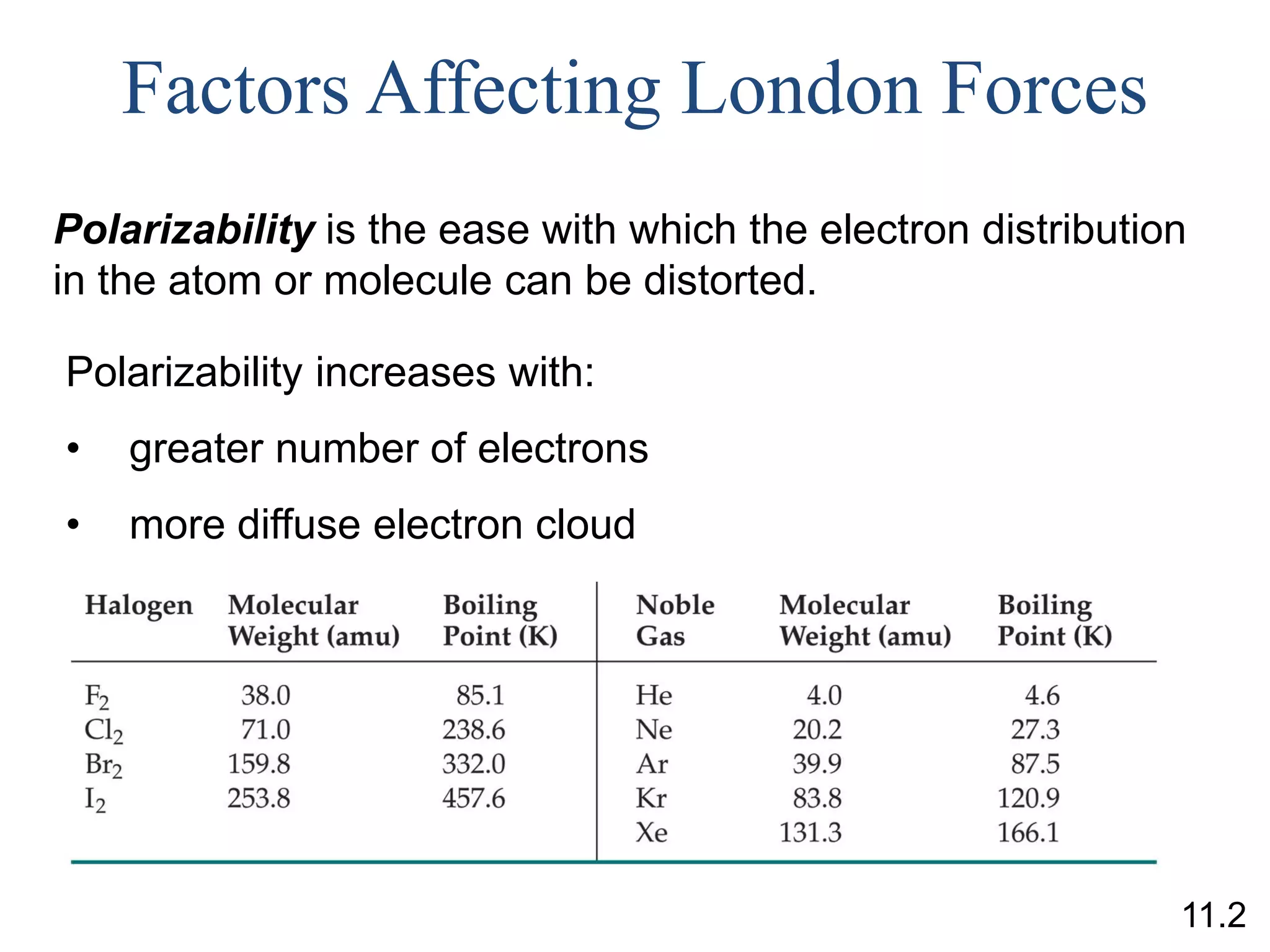
greater polarizability
greater intermolecular forces
higher melting (freezing) and boiling points, lower vapor pressure
melting point, strength of
intermolecular forces
] ~
a. highest boiling point
HBr, Kr, or Cl2 HBr > [Cl2>Kr]
c. lowest vapor pressure at 25ºC
Cl2, Br2, or I2 I2 < Br2 < Cl2](https://image.slidesharecdn.com/lecture2-3-230615171046-370007bb/75/Lecture-2-3-intermolecular-forzes-pptx-pdf-33-2048.jpg)

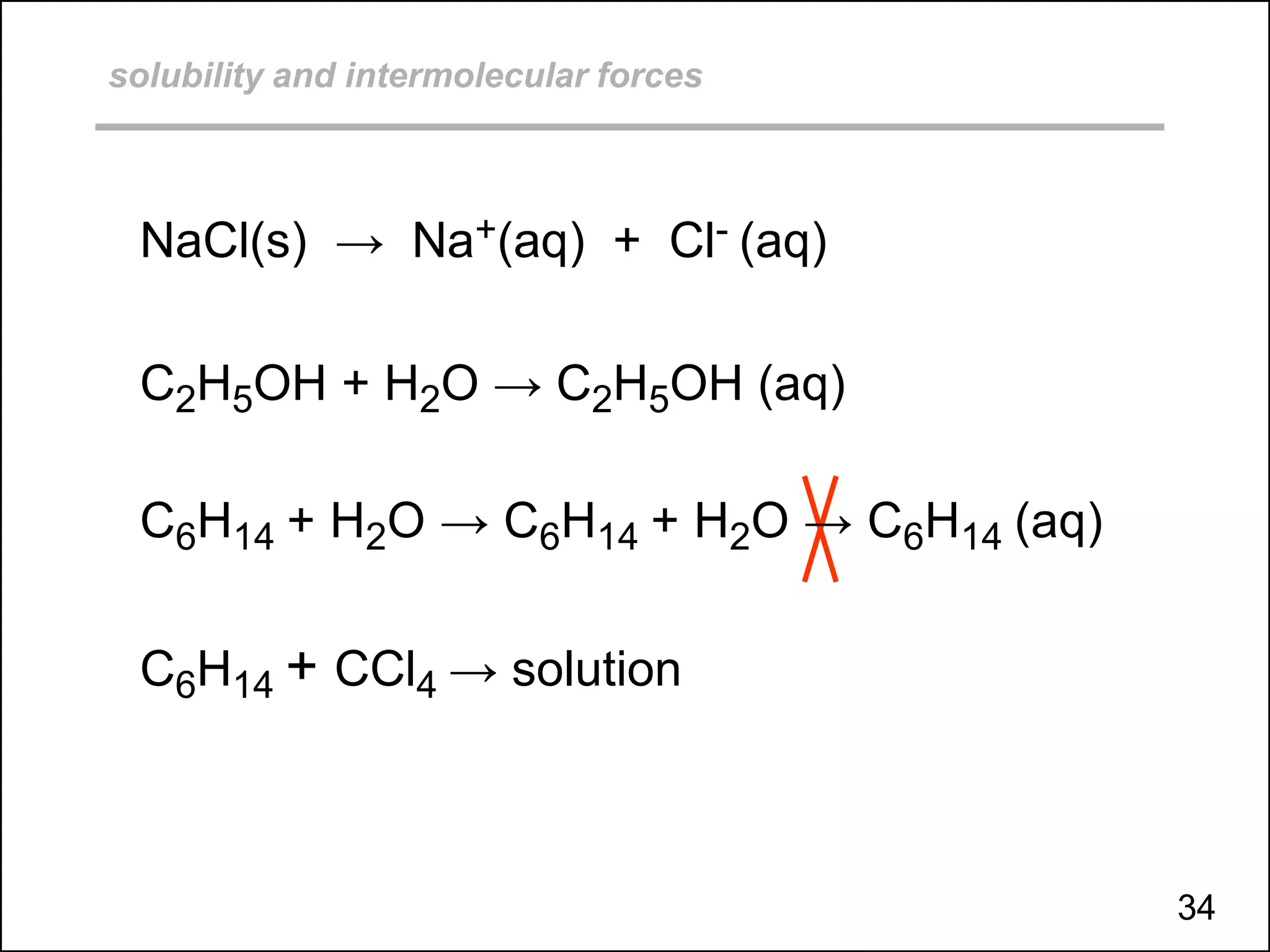

![solubility and intermolecular forces (ionic solids + polar solvent)
NaCl(s) → Na+(aq) + Cl- (aq)
[ion-ion] [ion-dipole]
36](https://image.slidesharecdn.com/lecture2-3-230615171046-370007bb/75/Lecture-2-3-intermolecular-forzes-pptx-pdf-41-2048.jpg)