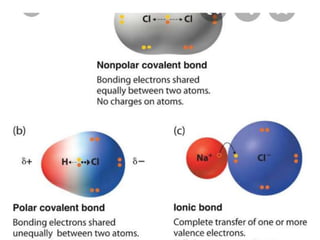
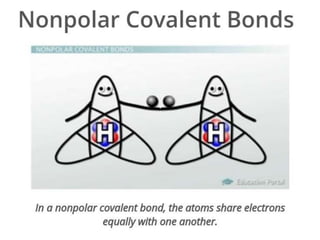
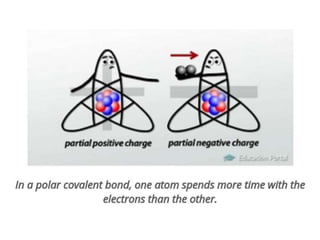
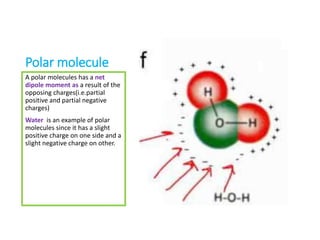
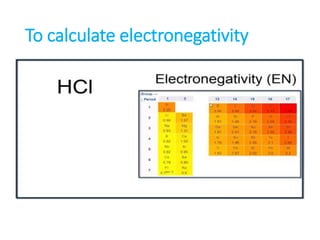
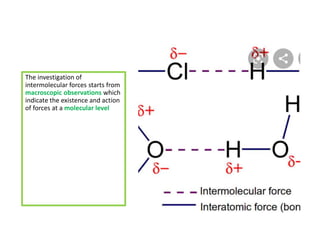
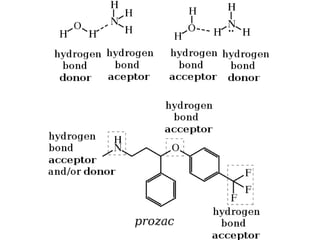
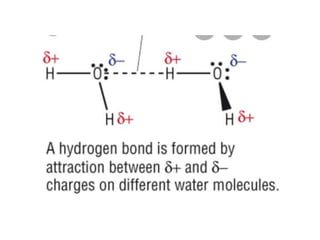
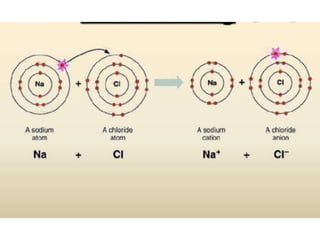
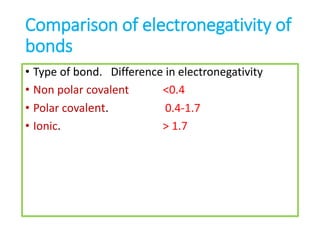
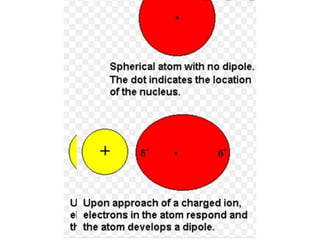
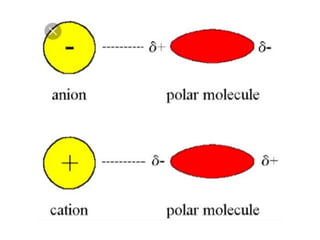
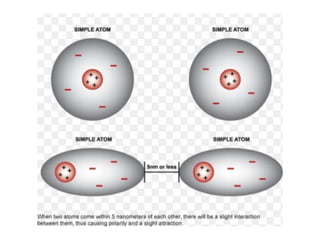
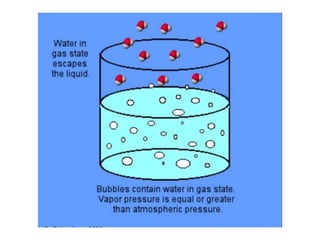
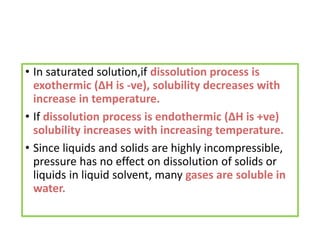
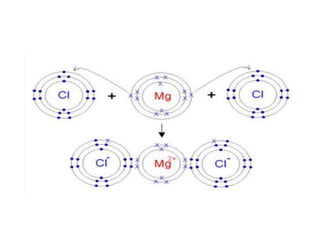
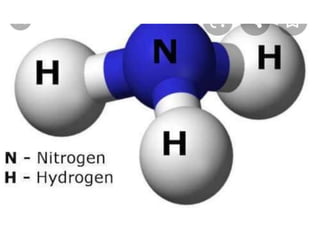
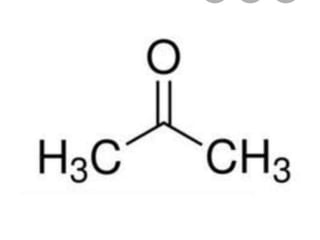
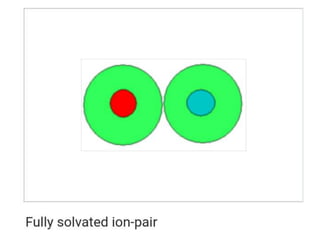
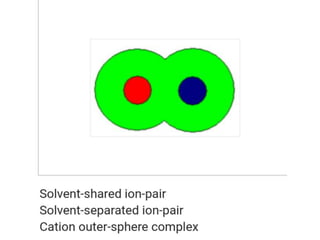
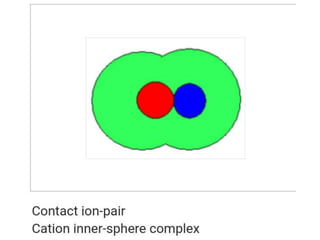
The document discusses different types of chemical bonds including polar and nonpolar bonds. It explains that polarity refers to differences in electronegativity between atoms in a bond. Polar bonds form when electrons are shared unequally, resulting in partial positive and negative charges on different parts of the molecule. Nonpolar bonds share electrons equally. The document also describes properties influenced by bond polarity like boiling point and solubility. It discusses hydrogen bonding, ionic bonding, and van der Waals forces as types of intermolecular forces.