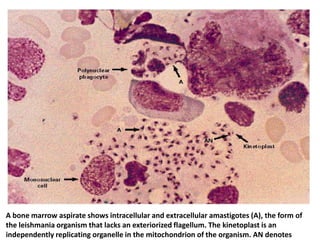
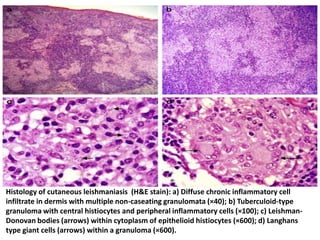
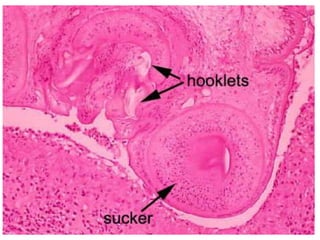
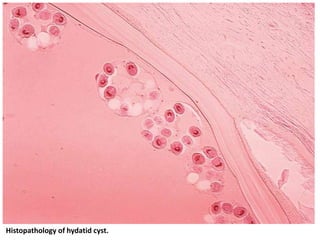
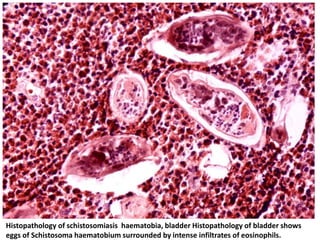
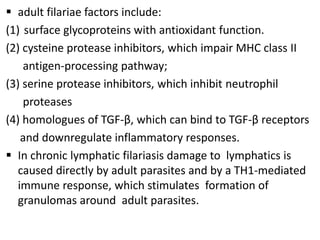
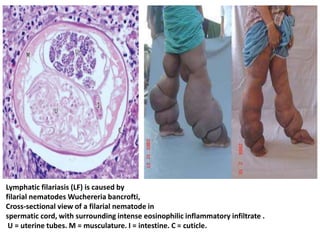
This document discusses various parasitic infections caused by protozoa and metazoa. It describes the life cycles and pathologies of malaria, caused by Plasmodium parasites transmitted by mosquitoes; leishmaniasis, caused by Leishmania parasites transmitted by sandflies; tapeworm infections such as cysticercosis caused by Taenia solium larvae; hydatid disease caused by Echinococcus granulosus; schistosomiasis caused by blood flukes of the genus Schistosoma transmitted by snails; and lymphatic filariasis caused by Wuchereria bancrofti transmitted by mosquitoes. For each parasite, it details the clinical manifestations, histopathology, and


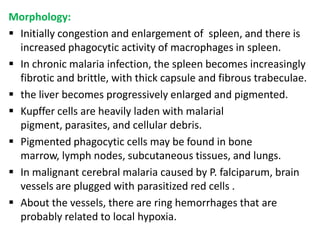
![[MALARIAL PARASITES IN BLOOD]. Note the presence of two chromatin dots in each
trophozoite inside red blood cells. Note also the two trophozoites in another red blood cell.
The presence of two chromatin dots and two trophozoites is a feature of P. falciparum.](https://image.slidesharecdn.com/infectiousdisease-p5-130219045756-phpapp01/85/Infectious-disease-p5-5-320.jpg)