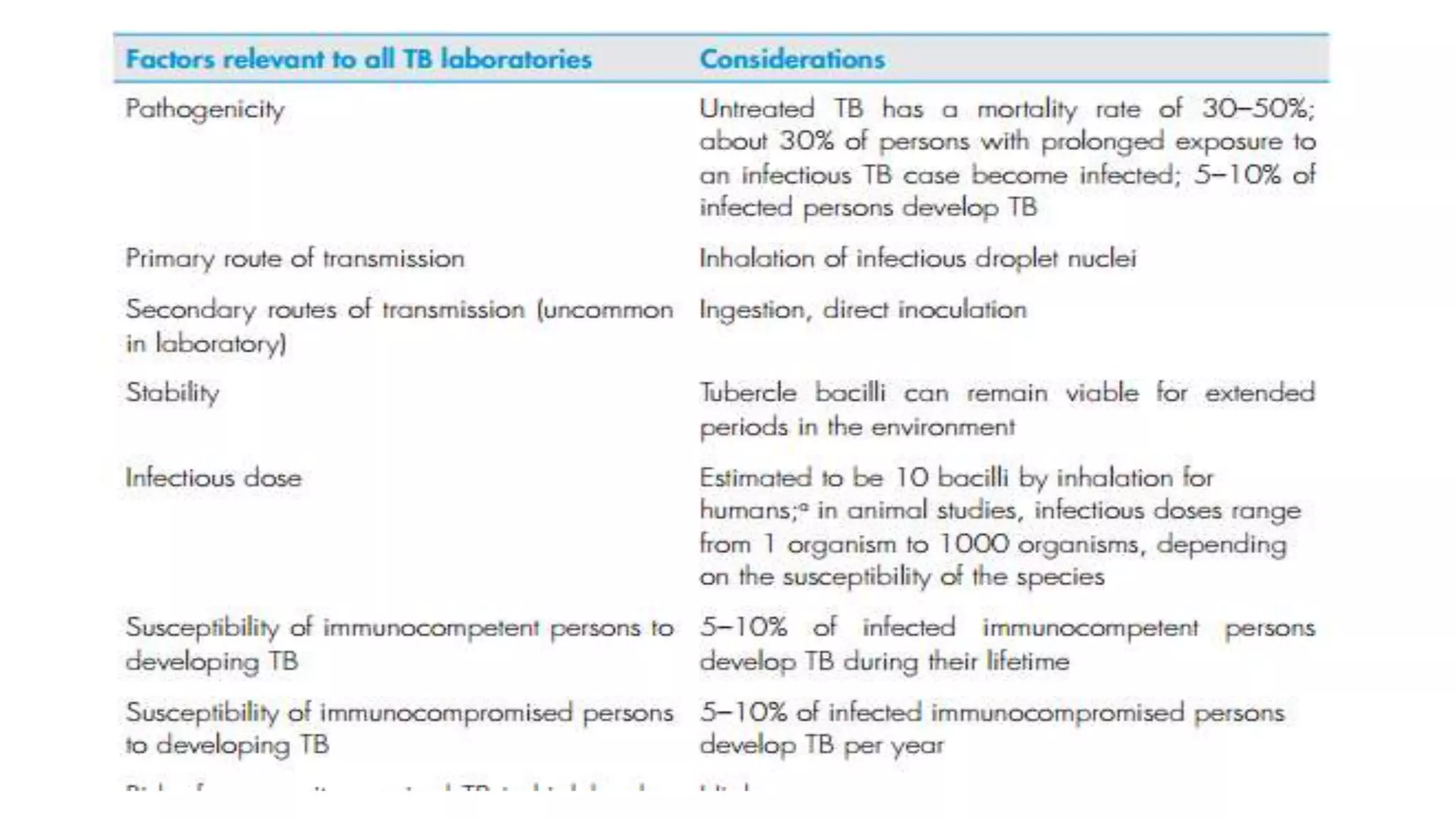
This document provides guidelines for infection control measures in TB laboratories of varying risk levels. It outlines biosafety measures, personal protective equipment, and procedures for handling specimens, cultures, and potential spills. The three key points are:
1) TB laboratories are classified as low, moderate, or high risk depending on the procedures performed and appropriate biosafety measures are outlined for each risk level.
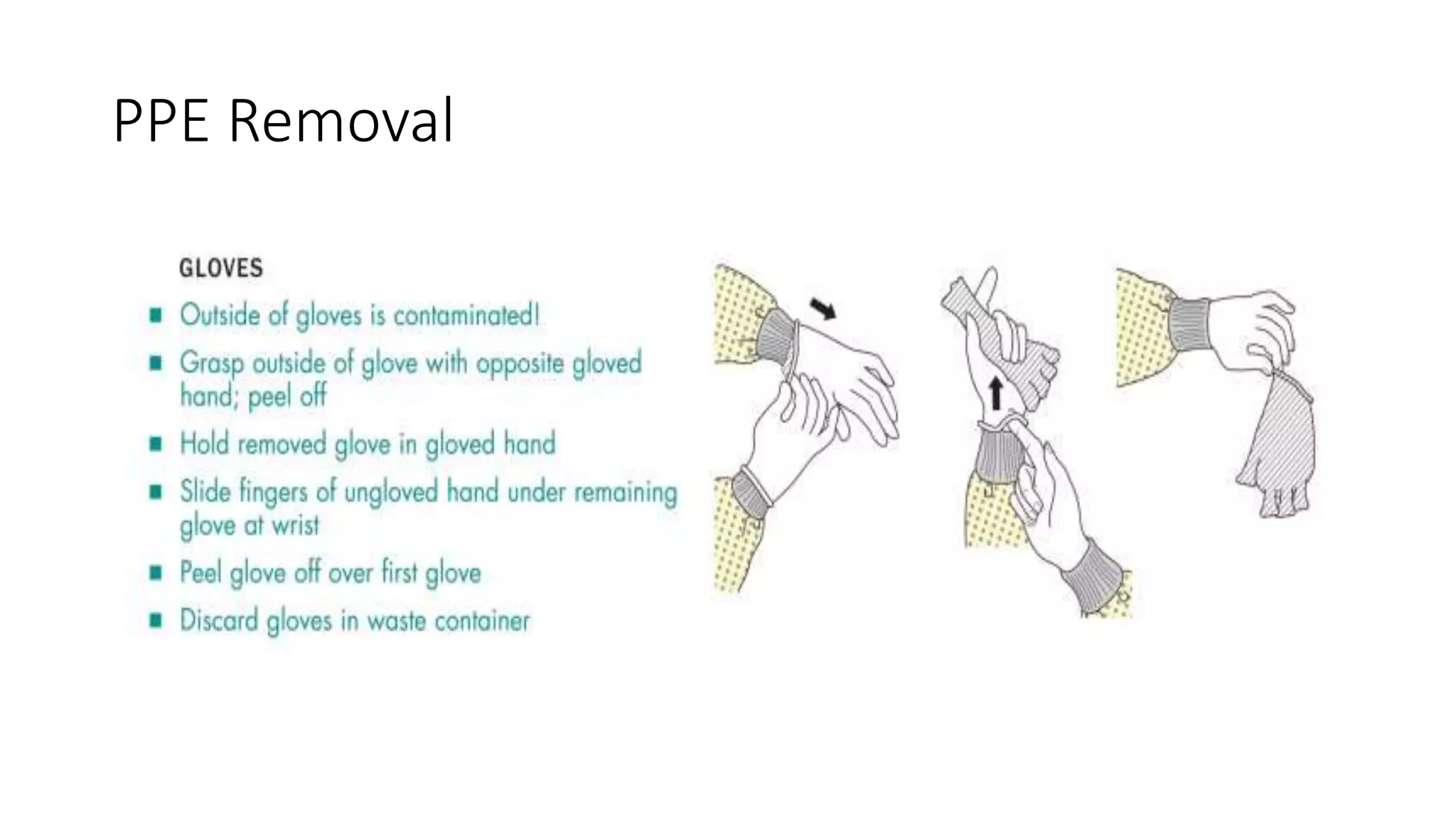
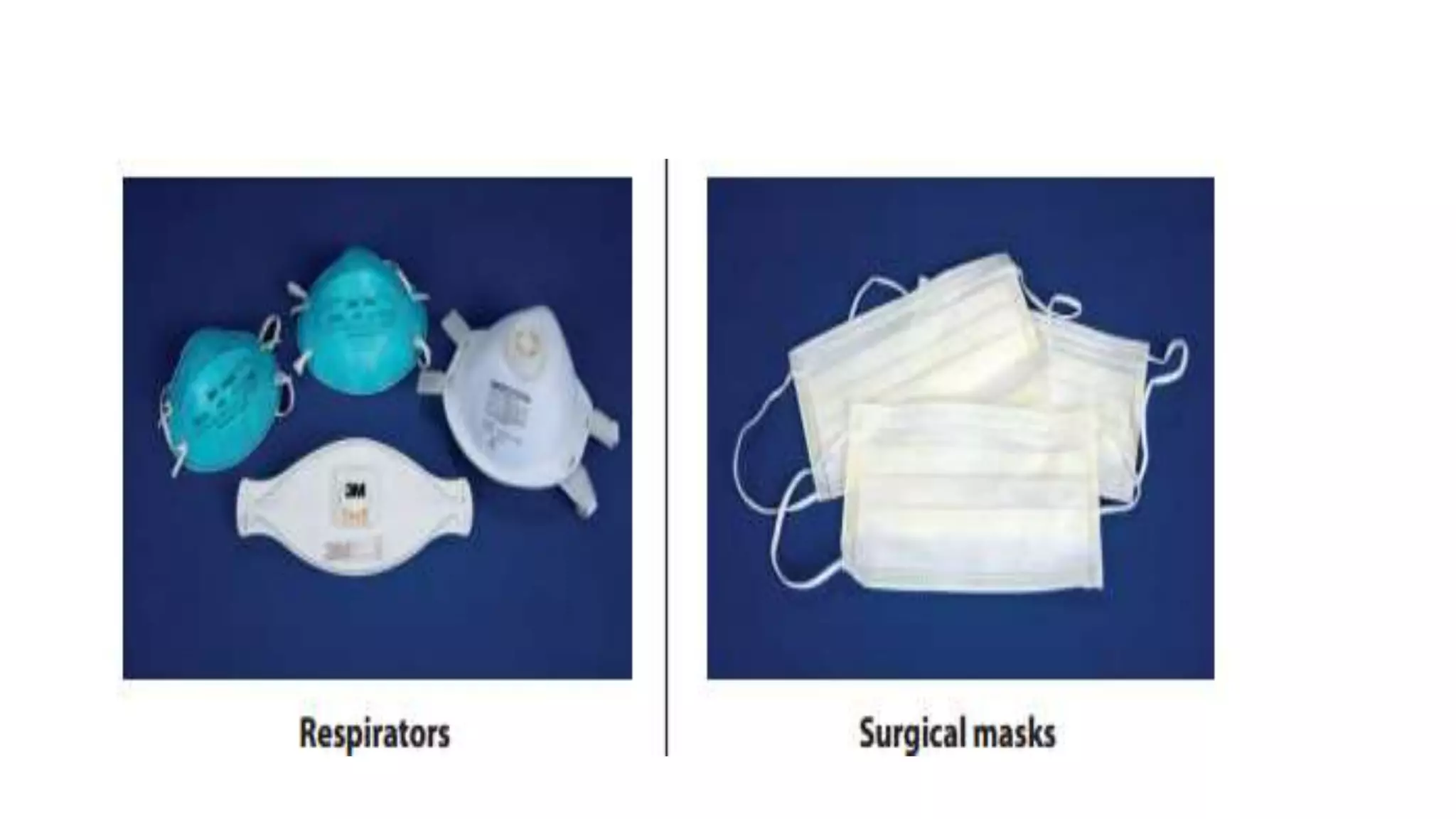
2) Personal protective equipment such as lab coats, gowns, gloves, and masks are described based on the task and risk level. Proper use and disposal of PPE is emphasized.
3) Detailed procedures are provided for handling potential spills or leaks of specimens and cultures, including containment, decont

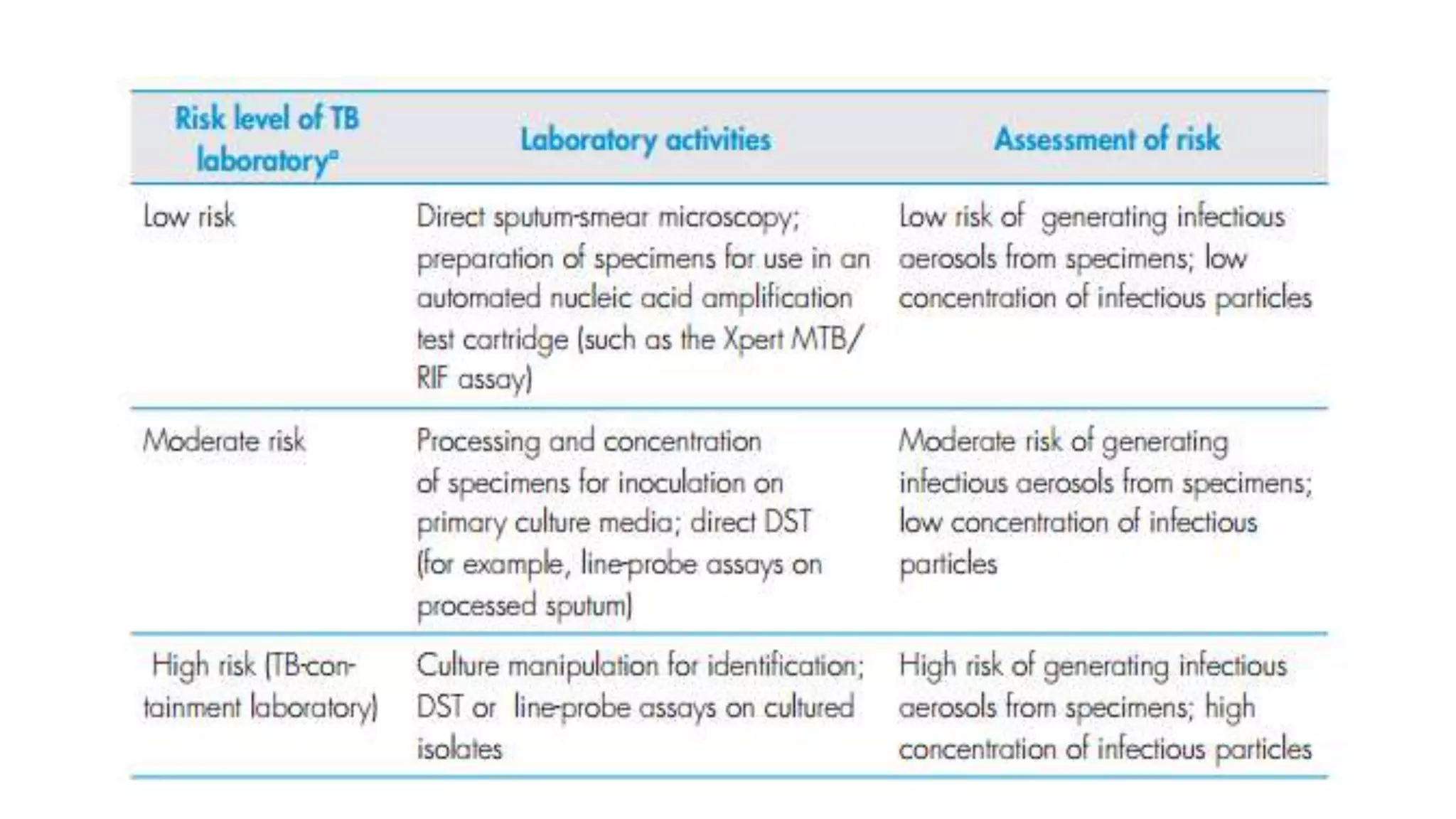
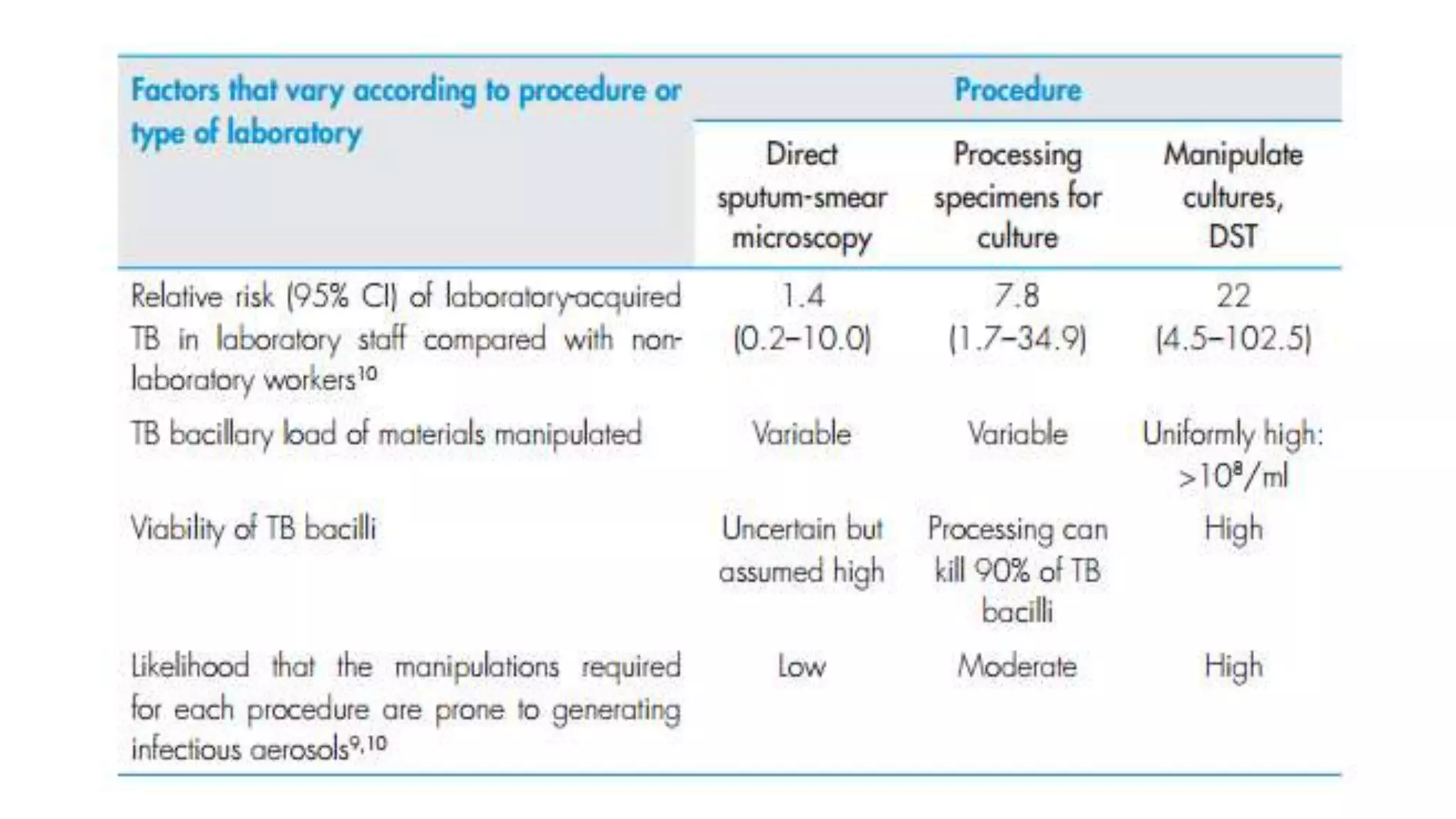




![Moderate Risk TB Laboratory
• Process specimens for inoculation on primary solid-culture media;
• Perform direct DST (for example, direct line-probe assays, Microscopic
observation drug susceptibility [MODS], Nitrate reductase assay
[NRA] on processed sputum](https://image.slidesharecdn.com/infectioncontrolmeasuresintblaboratory-180227102935/75/Infection-control-measures-in-tb-laboratory-9-2048.jpg)