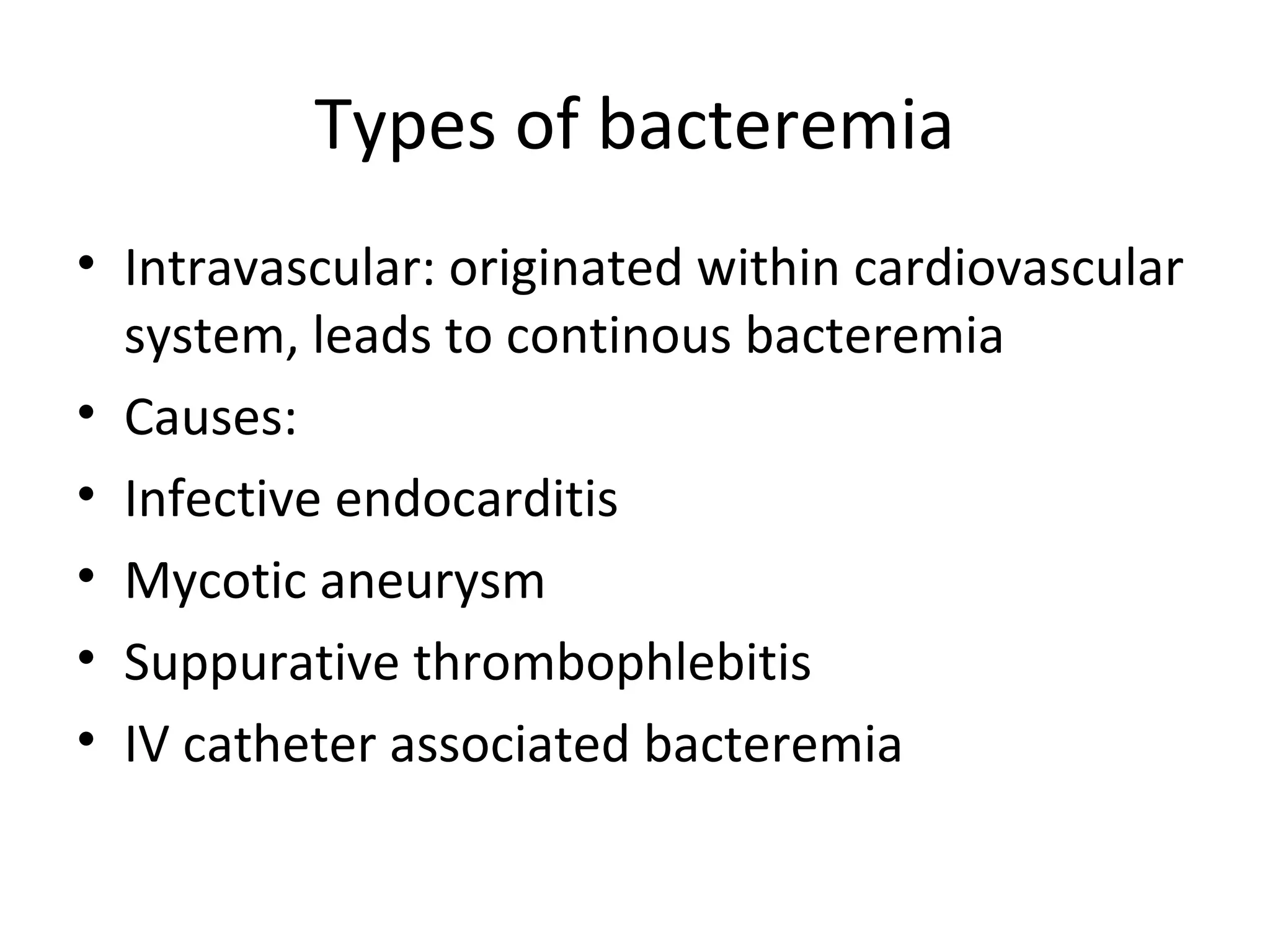
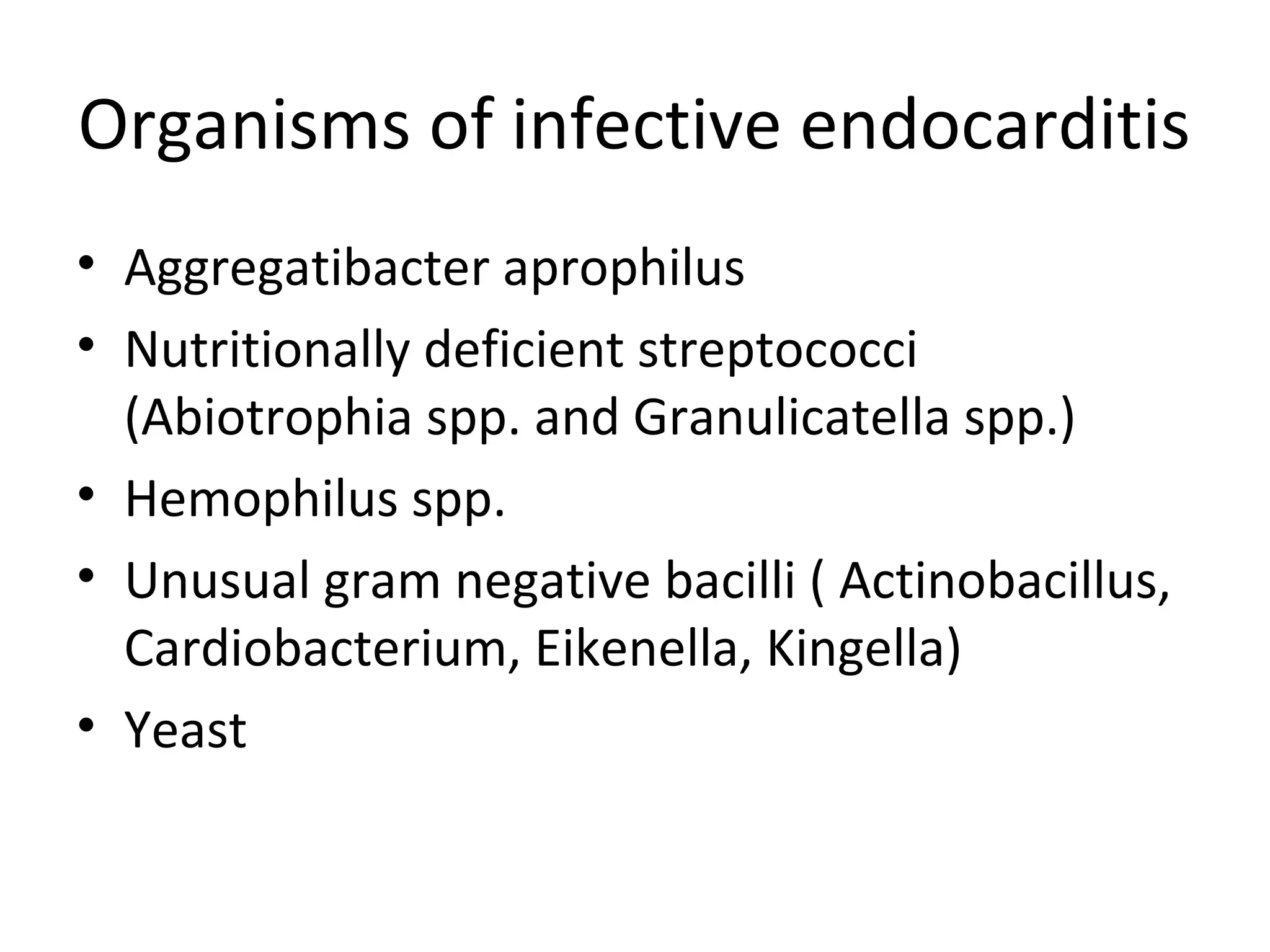
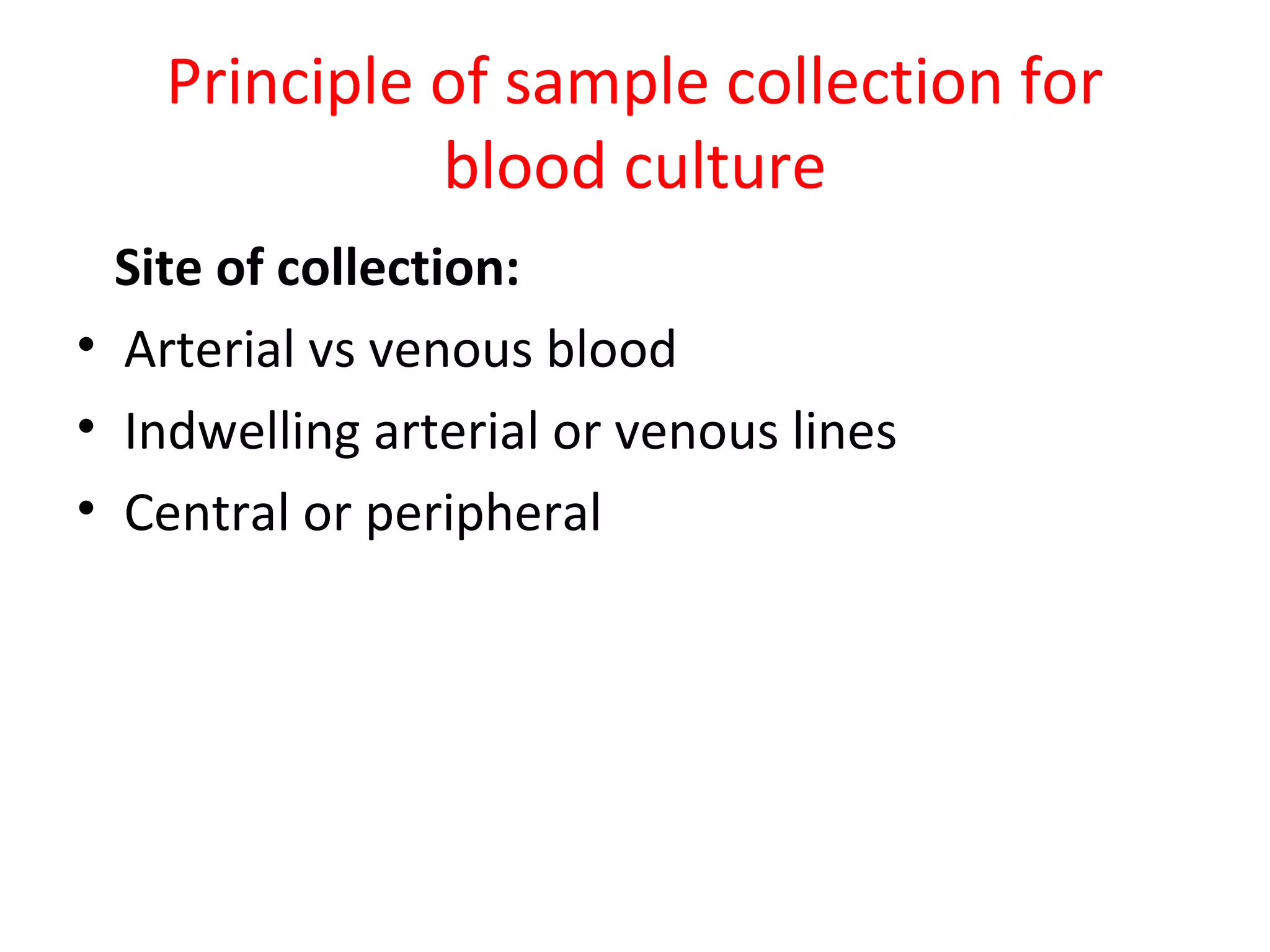
This document provides information about blood culture procedures. It defines blood culture and different types of bacteremia. Common organisms isolated from blood cultures are discussed. Proper collection of blood culture samples is important, including collecting the appropriate amount of blood from veins or catheters. Multiple blood cultures may increase the sensitivity of detection. Samples should be incubated aerobically and anaerobically so that a variety of organisms can be grown. Following proper collection and incubation procedures can help identify the causative microorganism in cases of bacteremia, fungemia or sepsis.