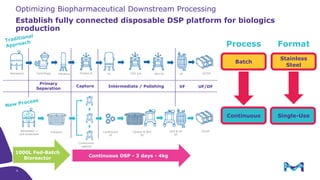
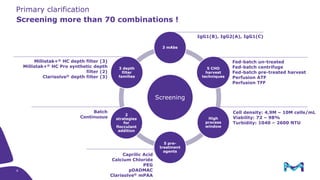
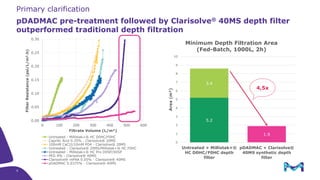
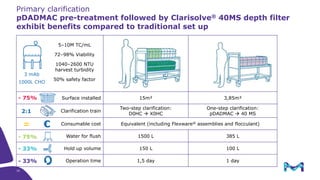
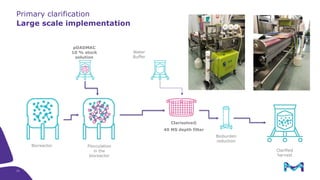
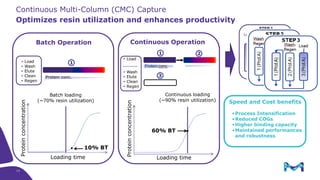
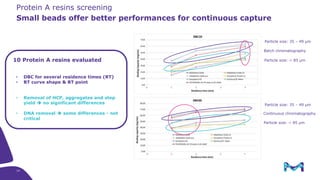
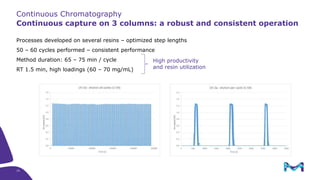
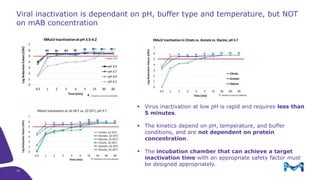
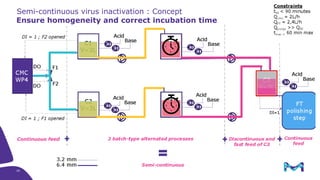
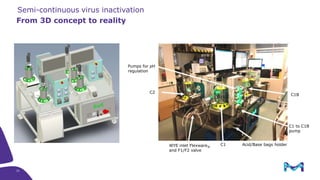
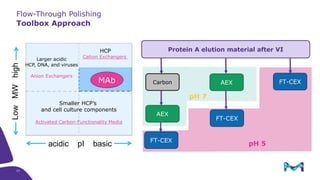
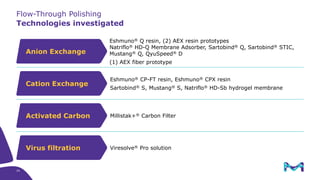
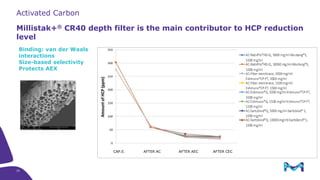
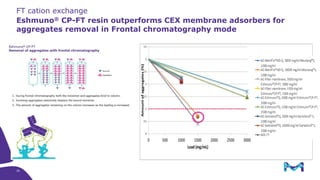
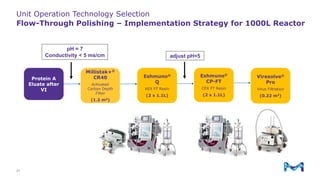
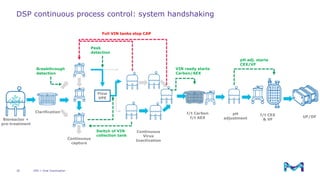
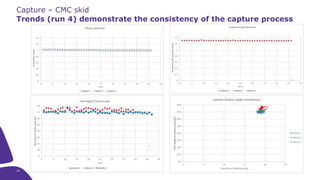
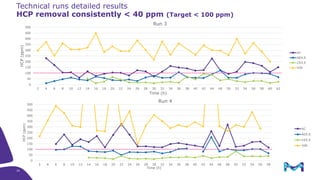
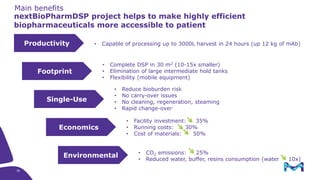
Merck KGaA, Darmstadt, Germany, is implementing a fully single-use, integrated monoclonal antibody biosimilars purification platform under the NextBiopharmDSP project, aimed at creating a more efficient and environmentally friendly downstream process for biopharmaceutical manufacturing. The project involves multiple industry and academic partners, and focuses on innovations such as continuous capture, viral inactivation, and flow-through downstream processing at a 1000L scale. Funded by the EU's Horizon 2020 program, the initiative seeks to enhance productivity, reduce costs, and increase accessibility of biopharmaceuticals to patients.