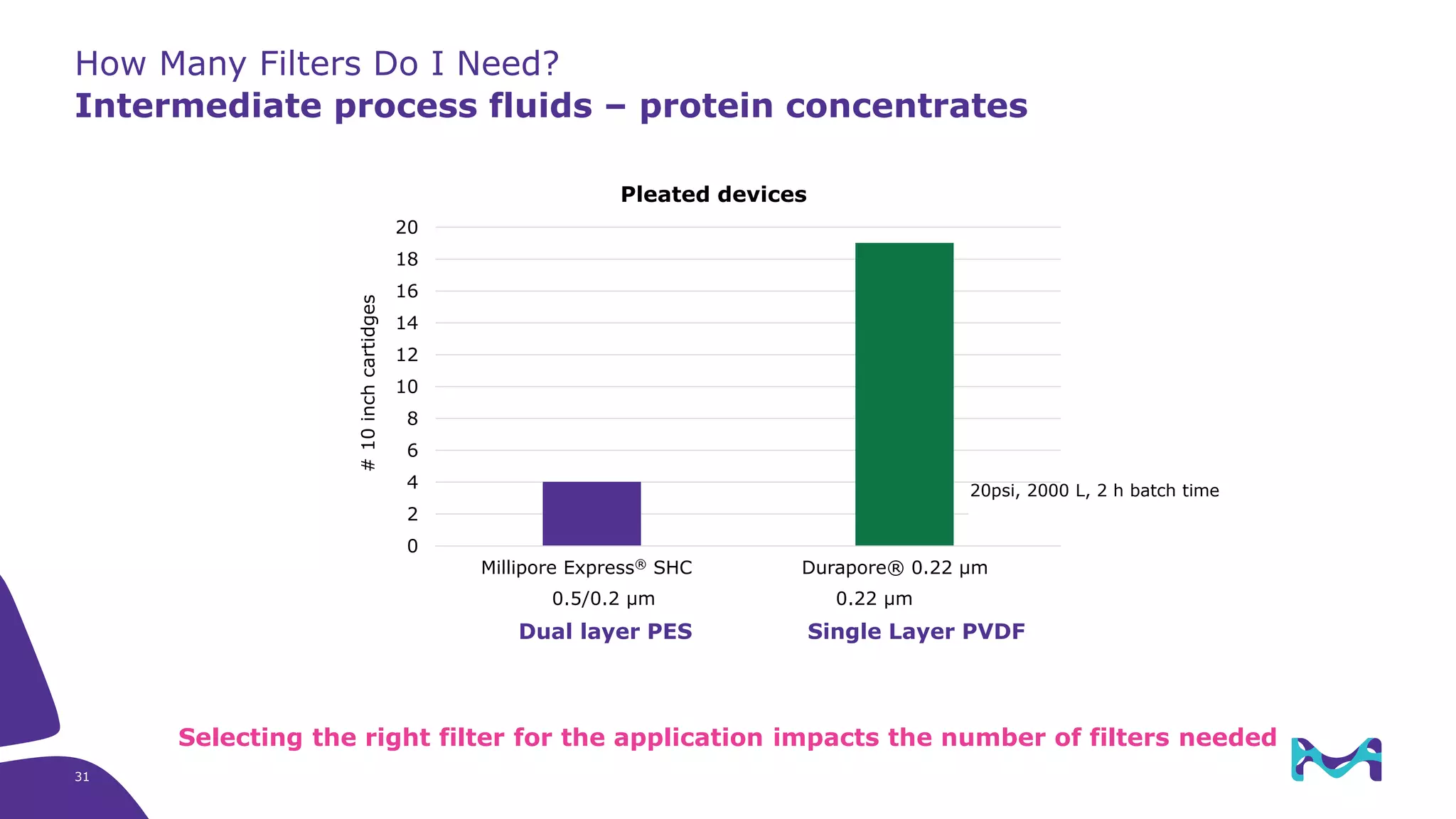
The document discusses filtration in biopharmaceutical processes, focusing on aseptic and bioburden reduction filters from Merck KGaA. It outlines the importance of selecting appropriate filters based on risk profiles at various process stages and details different types of membranes and devices optimized for specific applications. The content also highlights case studies and technological advancements to improve process efficiency and maintain product sterility.


























![Ashby-Leon, et. al. (2018). Improve Process Efficiency in Bioprocess Streams by Prefiltration Optimization and Bioburden Reduction.
ASTM International. (2005). ASTM F838-05, Standard Test Method for Determining Bacterial Retention of Membrane Filters Utilized for Liquid Filtration. Retrieved
from www.astm.org
EMD Millipore Corporation, a division of Merck KGaA, Damstadt Germany. (2013). Simplified, efficient sizing of sterilizing-grade normal flow filters for buffer
solutions,” Technical Brief TB4462EN00. Retrieved from www.millipore.com
FDA. (2004). Guidance for Industry. Sterile Drug Products Produced by Aseptic Processing — Current Good Manufacturing Practice. Retrieved from
https://www.fda.gov/downloads/Drugs/Guidances/ucm070342.pdf
Mahler, et. al. (2010) Adsorption behavior of a surfactant and a monoclonal antibody to sterilizing-grade filters. Journal of Pharmaceutical Sciences,
Vol. 99, No. 6
Parenteral Drug Association. (2008). Sterilizing filtration of liquids. Technical Report No. 26. Retrieved from www.pda.org
Pitt, Aldo M. (1987). The Nonspecific Protein Binding of Polymeric Microporous Membranes
Roche-Lentine, K. (2018, October). Strategies to address bioburden control in downstream processing [Webinar]. Retrieved
from https://www.emdmillipore.com/US/en/support/webinars-upcoming-and-on-demand/Oimb.qB.l3kAAAFfPXRc27th,nav
Zhou, et. al. (2008). Non-Specific Binding and Saturation of Polysorbate-20 with Aseptic Filter Membranes for Drug Substance and Drug Product during mAb
Production. Journal of Membrane Science
Find Your Filter. What’s best for your process?
Recommended Reading & References
36](https://image.slidesharecdn.com/findyourfilterslideshare-181207090041/75/Find-your-filter-What-s-best-for-your-process-36-2048.jpg)
