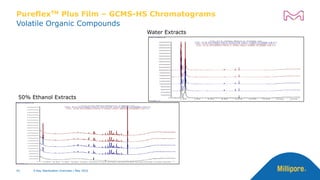
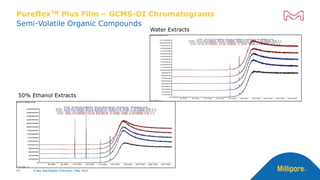
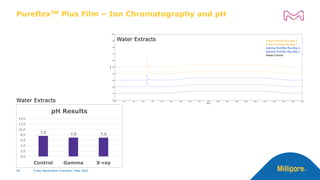
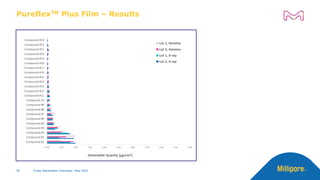
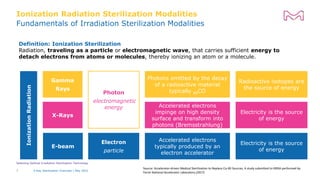
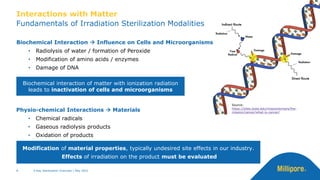
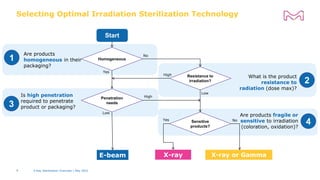
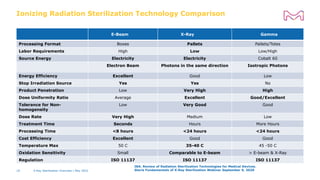
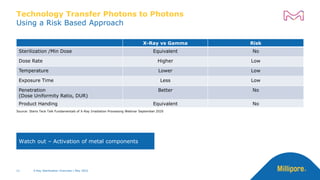
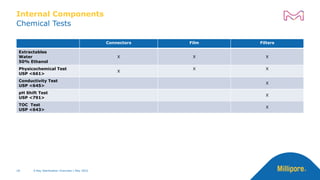
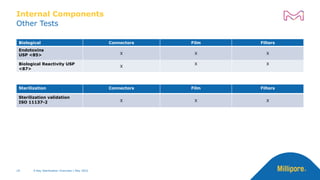
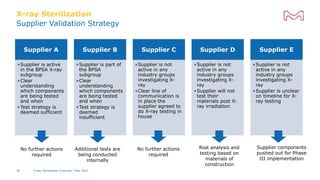
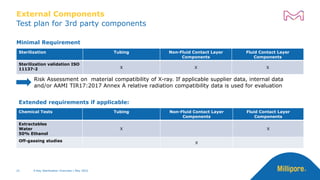
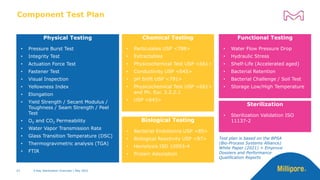
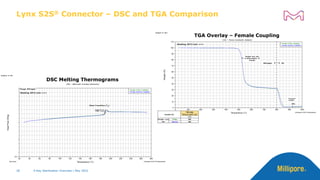
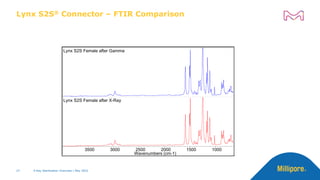
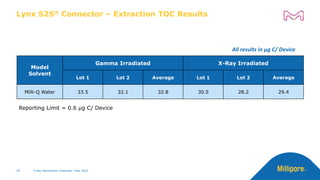
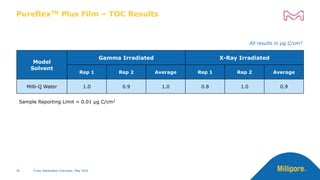
The document discusses testing done to qualify the use of x-ray sterilization for a Lynx S2S connector. Physical, chemical, and biological tests were performed on connectors that underwent either x-ray or gamma sterilization. Test results showed comparable extractable levels, thermal properties, and chromatographic profiles between the two sterilization methods. This provides evidence that x-ray sterilization is a suitable alternative to gamma sterilization for this connector.











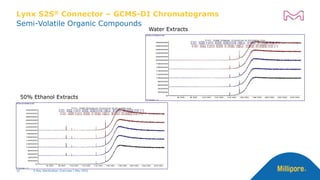
![Lynx S2S® Connector – HPLC Chromatograms
214
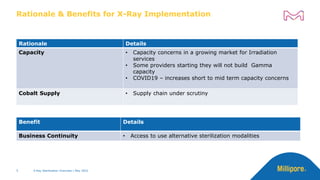
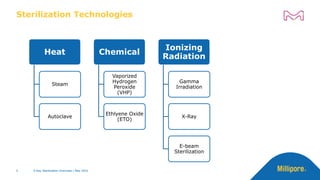
nm
Absorbance
[AU]
-0.02
0.00
0.02
0.04
0.06
0.08
0.10
0.12
0.14
0.16
0.18
0.20
Time (minutes)
0.00 1.00 2.00 3.00 4.00 5.00 6.00 7.00 8.00 9.00 10.00 11.00 12.00 13.00 14.00 15.00 16.00 17.00 18.00 19.00 20.00
X-Ray Lot 2
X-Ray Lot 1
Gamma Lot 2
Gamma Lot 1
Water Blank
214
nm
Absorbance
[AU]
-0.02
0.00
0.02
0.04
0.06
0.08
0.10
0.12
0.14
0.16
0.18
0.20
Time (minutes)
0.00 1.00 2.00 3.00 4.00 5.00 6.00 7.00 8.00 9.00 10.00 11.00 12.00 13.00 14.00 15.00 16.00 17.00 18.00 19.00 20.00
X-Ray Lot 2
X-Ray Lot 1
Gamma Lot 2
Gamma Lot 1
50% EtOH Blank
Water Extracts
50% Ethanol Extracts
X-Ray Sterilization Overview | May 2022
30](https://image.slidesharecdn.com/webinarpresentationrisk-basedqualificationofx-raysterilizationmay05final-220506152255-ab5d3294/85/Risk-Based-Qualification-of-X-Ray-Sterilization-for-Single-Use-Systems-30-320.jpg)





![214
nm
Absorbance
[AU]
0.0000
0.0096
0.0192
0.0288
0.0384
0.0480
0.0576
0.0672
0.0768
0.0864
0.0960
Time (minutes)
0.00 1.00 2.00 3.00 4.00 5.00 6.00 7.00 8.00 9.00 10.00 11.00 12.00 13.00 14.00 15.00 16.00 17.00 18.00 19.00 20.00
PureflexTM Plus Film – HPLC Chromatograms
41
X-Ray PureFlex Plus Rep-2
X-Ray PureFlex Plus Rep-1
Gamma PureFlex Plus Rep-2
Gamma PureFlex Plus Rep-1
Water Control
214
nm
Absorbance
[AU]
-0.025
0.000
0.025
0.050
0.075
0.100
0.125
0.150
0.175
0.200
0.225
0.250
Time (minutes)
0.00 1.00 2.00 3.00 4.00 5.00 6.00 7.00 8.00 9.00 10.00 11.00 12.00 13.00 14.00 15.00 16.00 17.00 18.00 19.00 20.00
X-Ray PureFlex Plus Rep-2
X-Ray PureFlex Plus Rep-1
Gamma PureFlex Plus Rep-2
Gamma PureFlex Plus Rep-1
50% Ethanol Control
Water Extracts
50% Ethanol Extracts
41 X-Ray Sterilization Overview | May 2022
X-Ray Sterilization Overview | May 2022](https://image.slidesharecdn.com/webinarpresentationrisk-basedqualificationofx-raysterilizationmay05final-220506152255-ab5d3294/85/Risk-Based-Qualification-of-X-Ray-Sterilization-for-Single-Use-Systems-41-320.jpg)