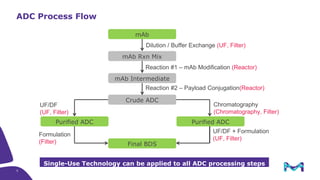
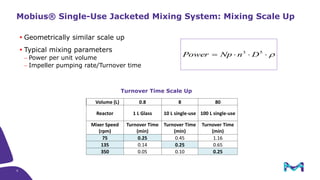
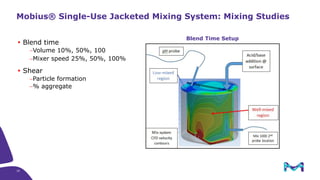
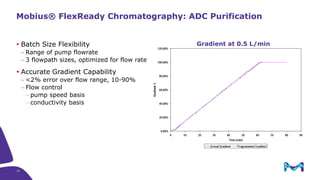
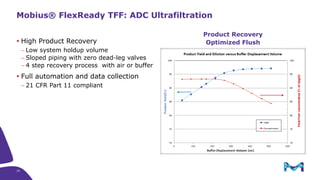
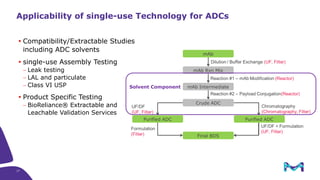
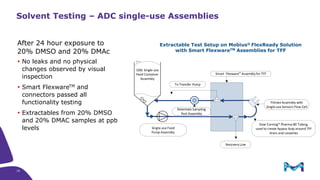
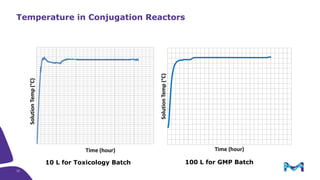
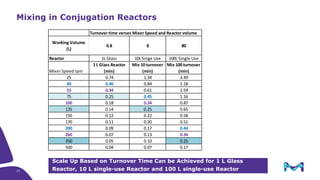
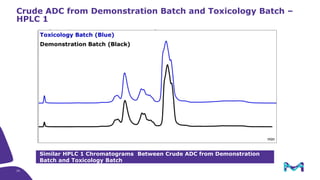
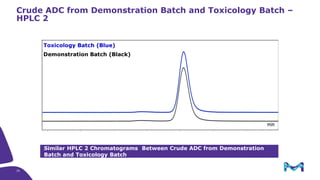
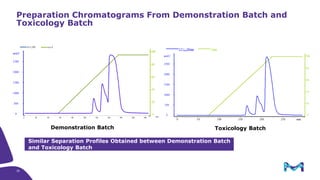
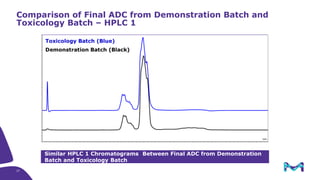
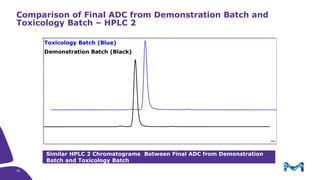
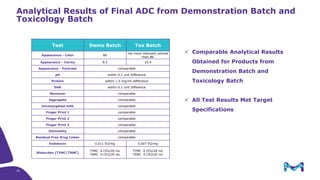
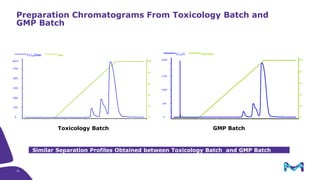
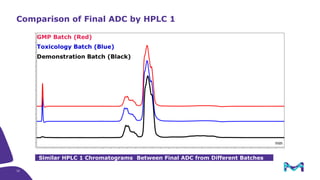
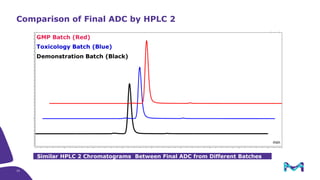
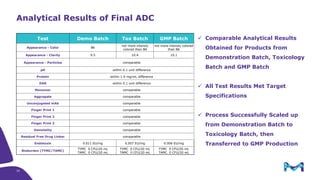
The document discusses the growth of the antibody drug conjugates (ADC) market, projecting a CAGR of 21.82% from 2017-2022 due to increasing cancer cases and a robust pipeline of ADC drugs. It outlines the advantages of single-use technology in ADC production, including safety, efficiency, and cost-effectiveness, and details various Mobius® systems for ADC processing, such as mixing and chromatography. Additionally, it emphasizes successful scaling up of bioprocess chemistry for ADC-X, aiming for consistency and high yield for clinical trials.