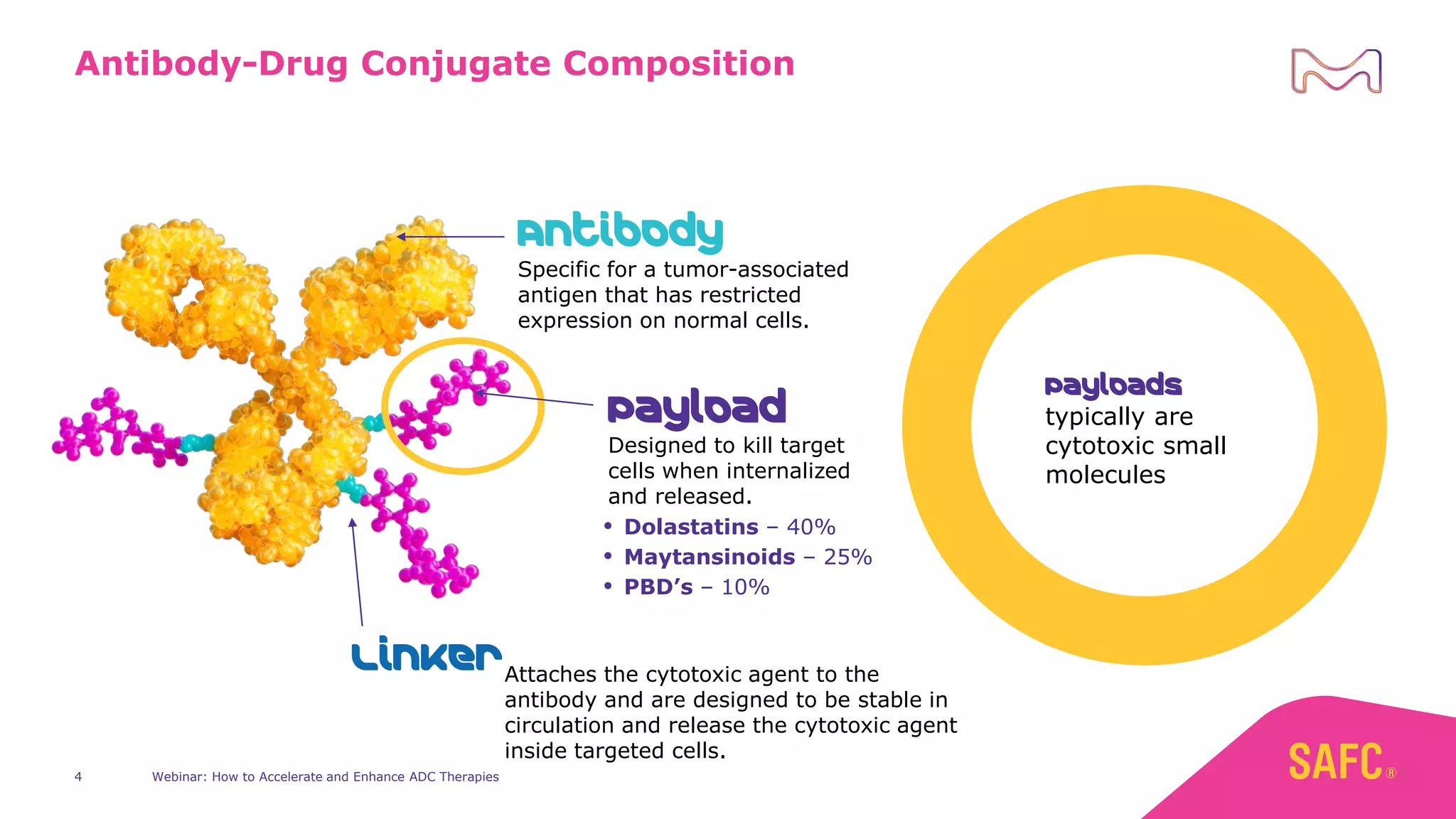
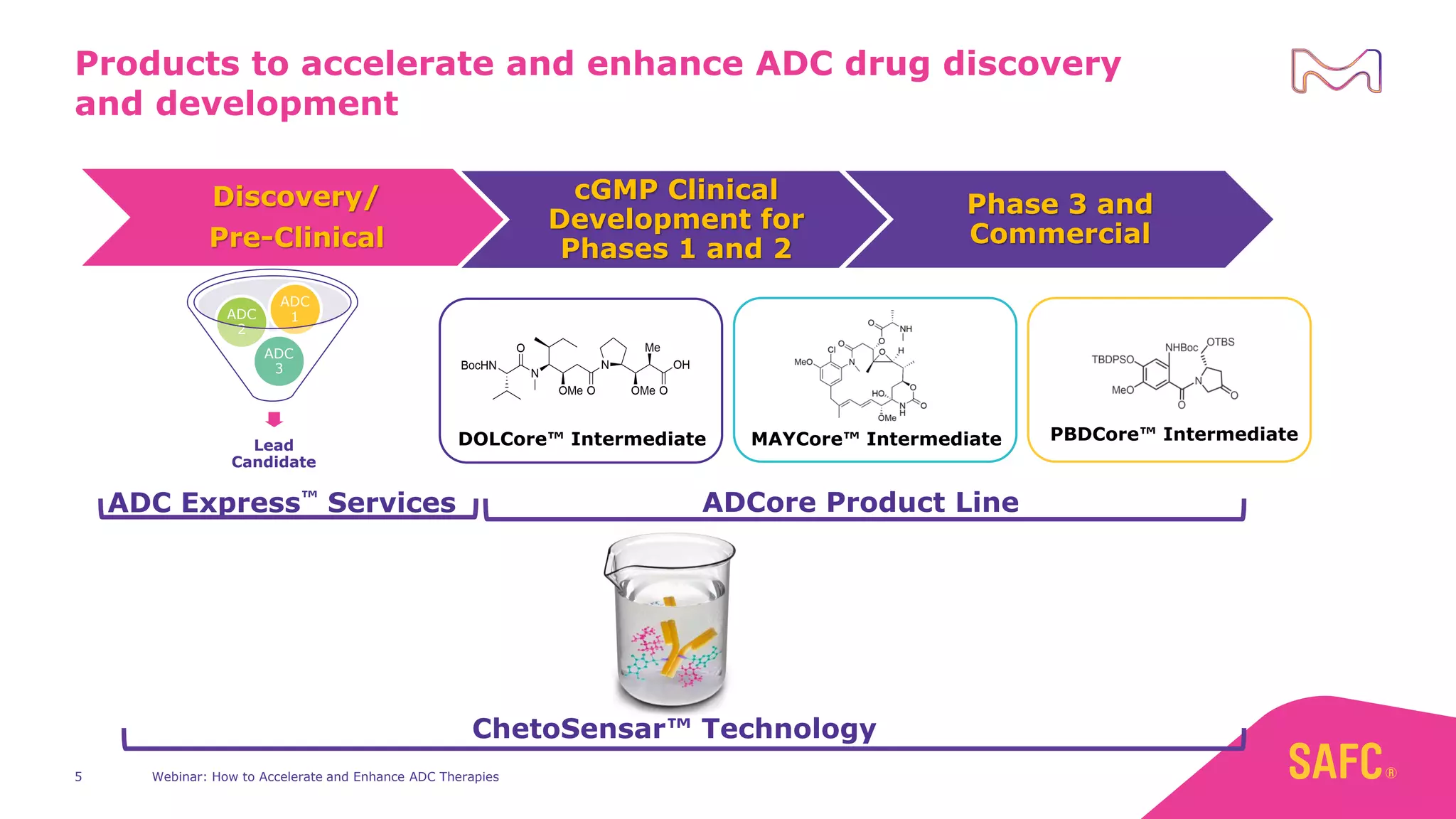
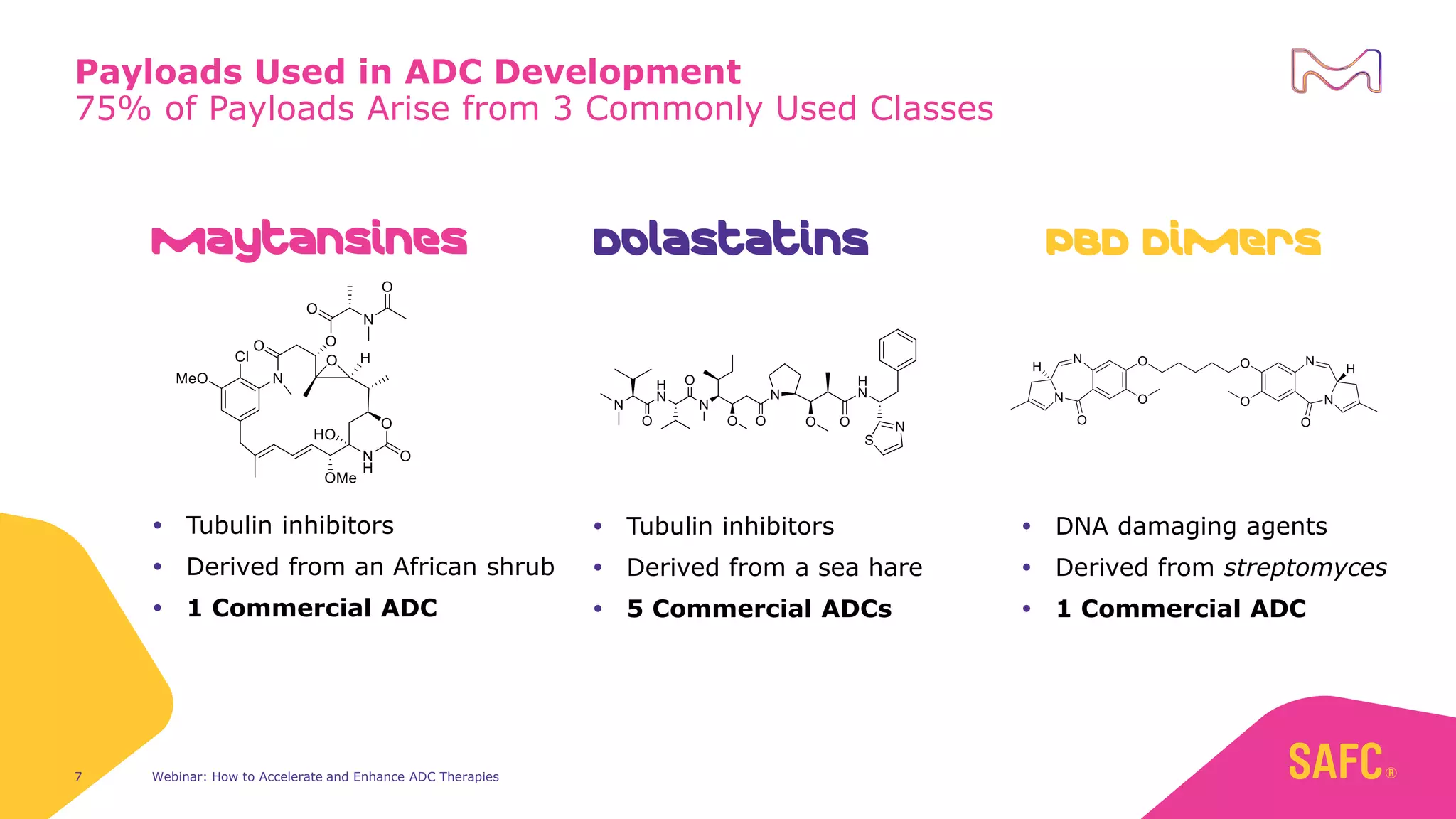
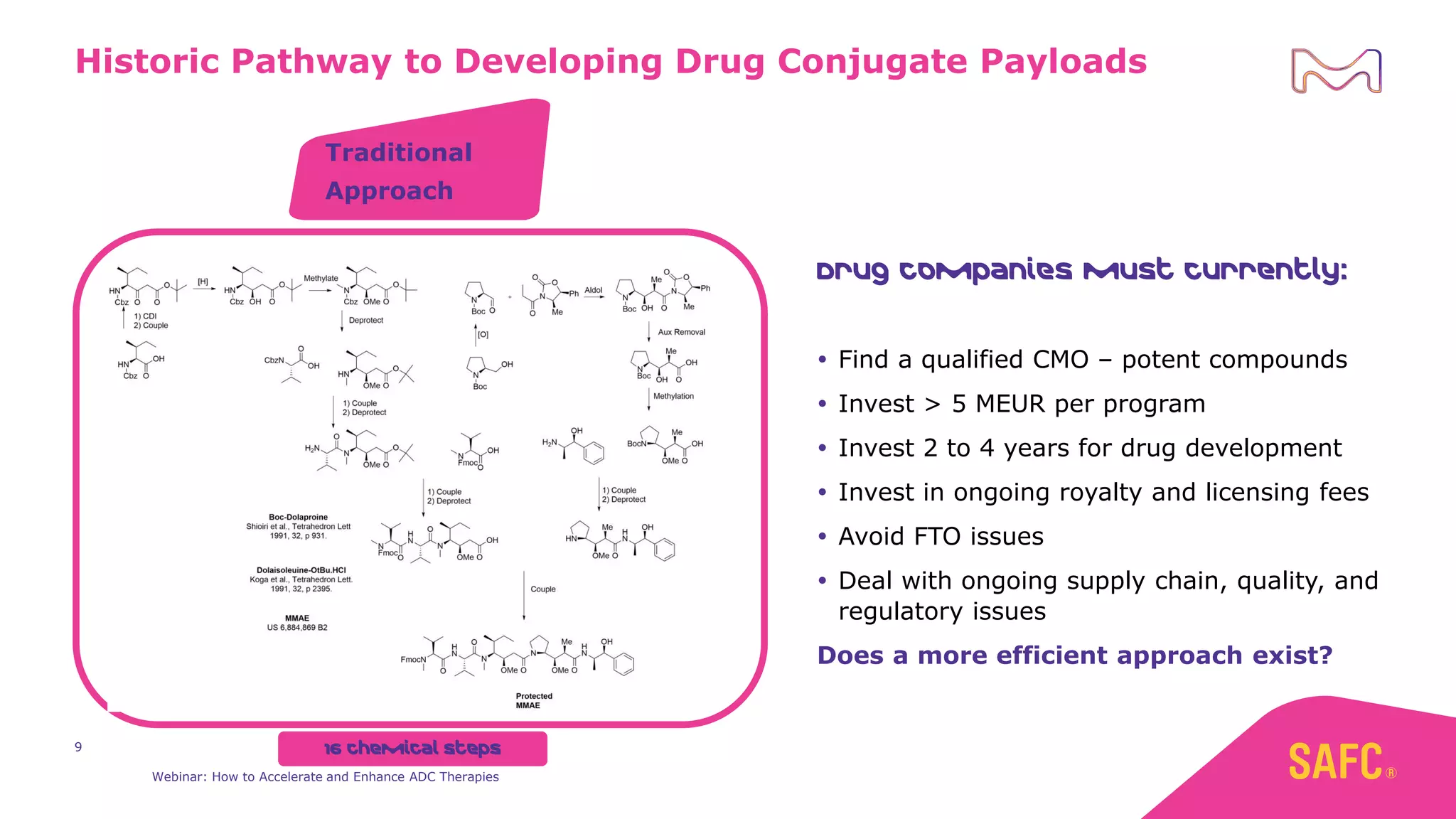
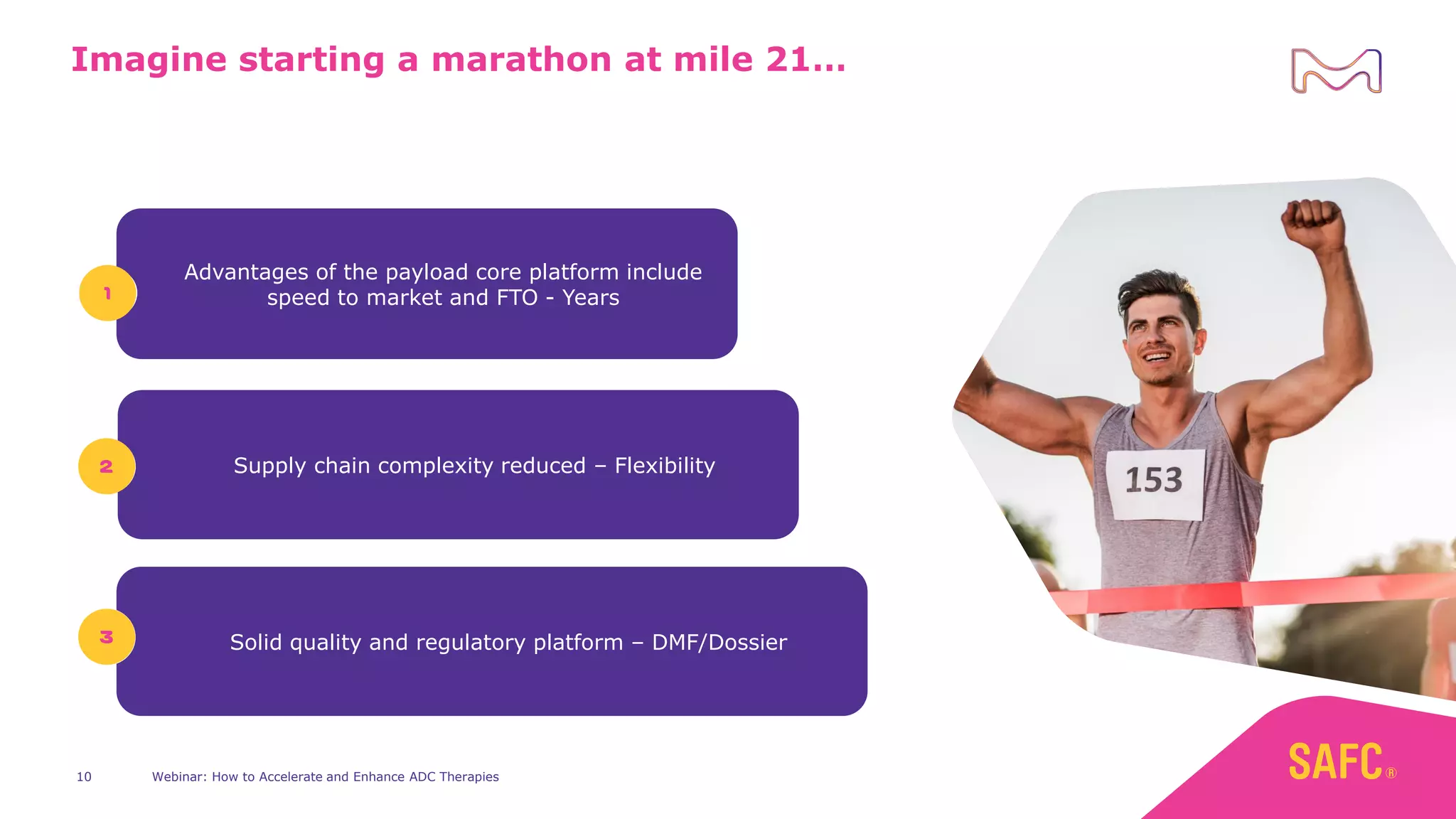
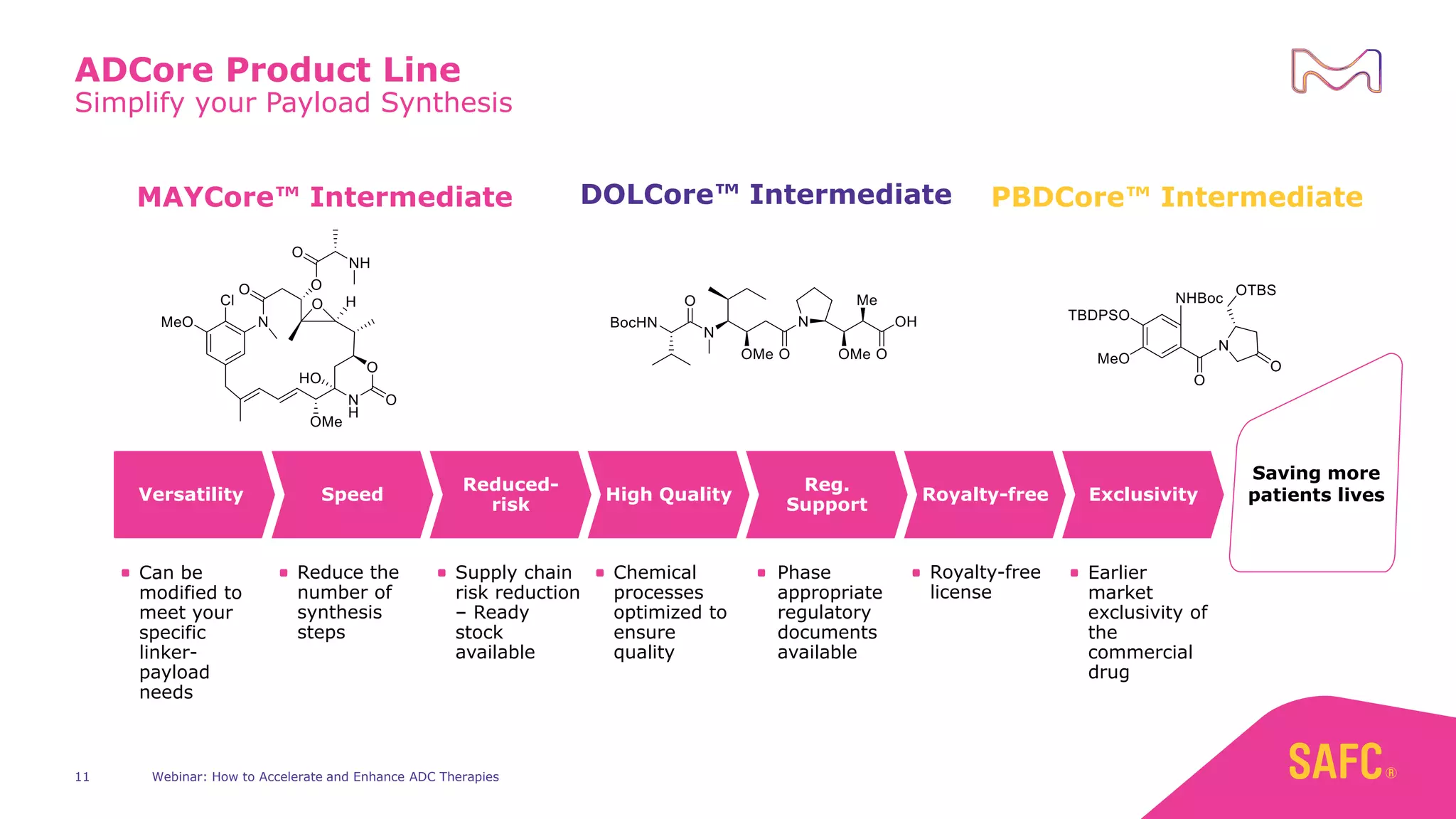
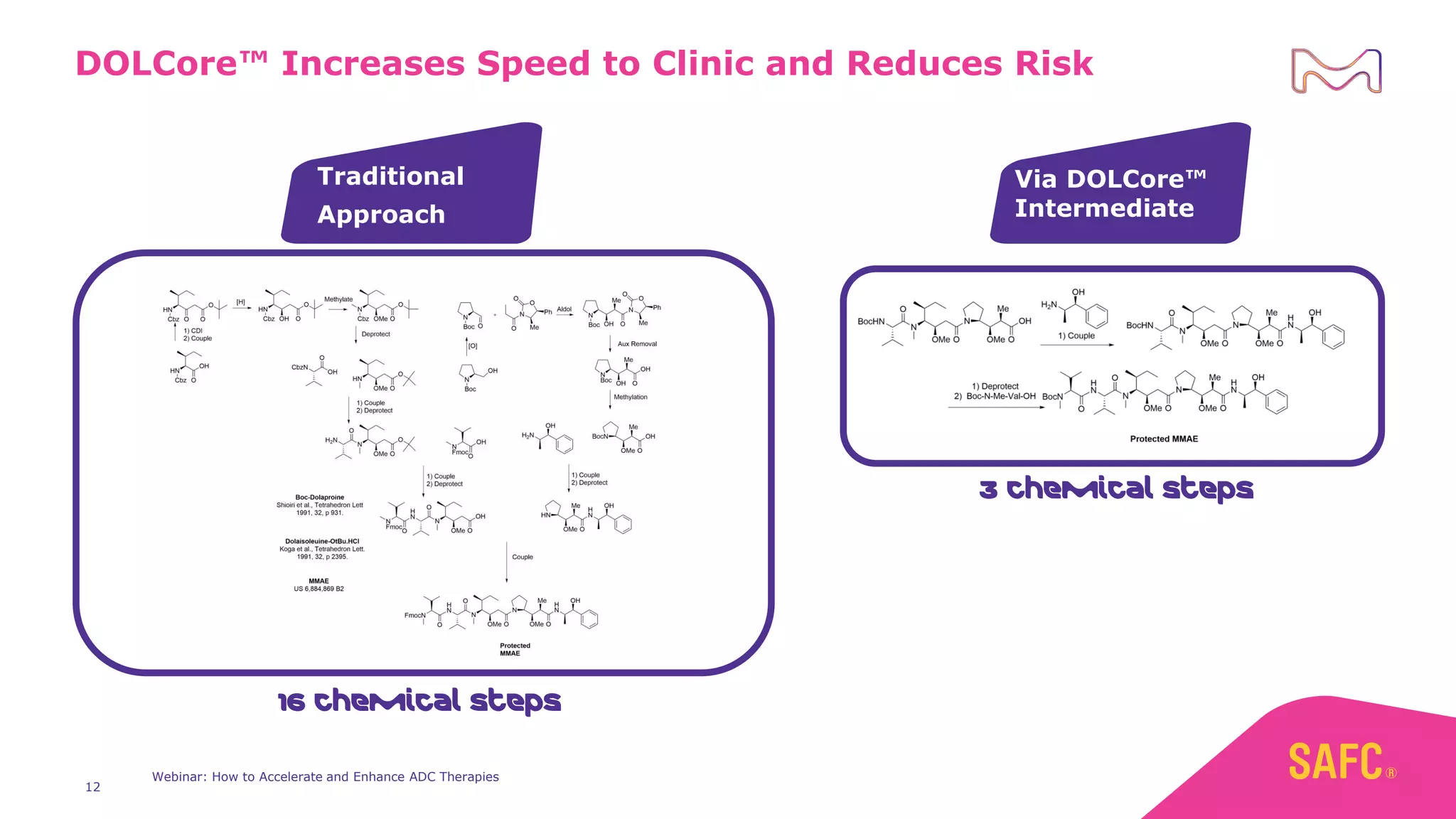
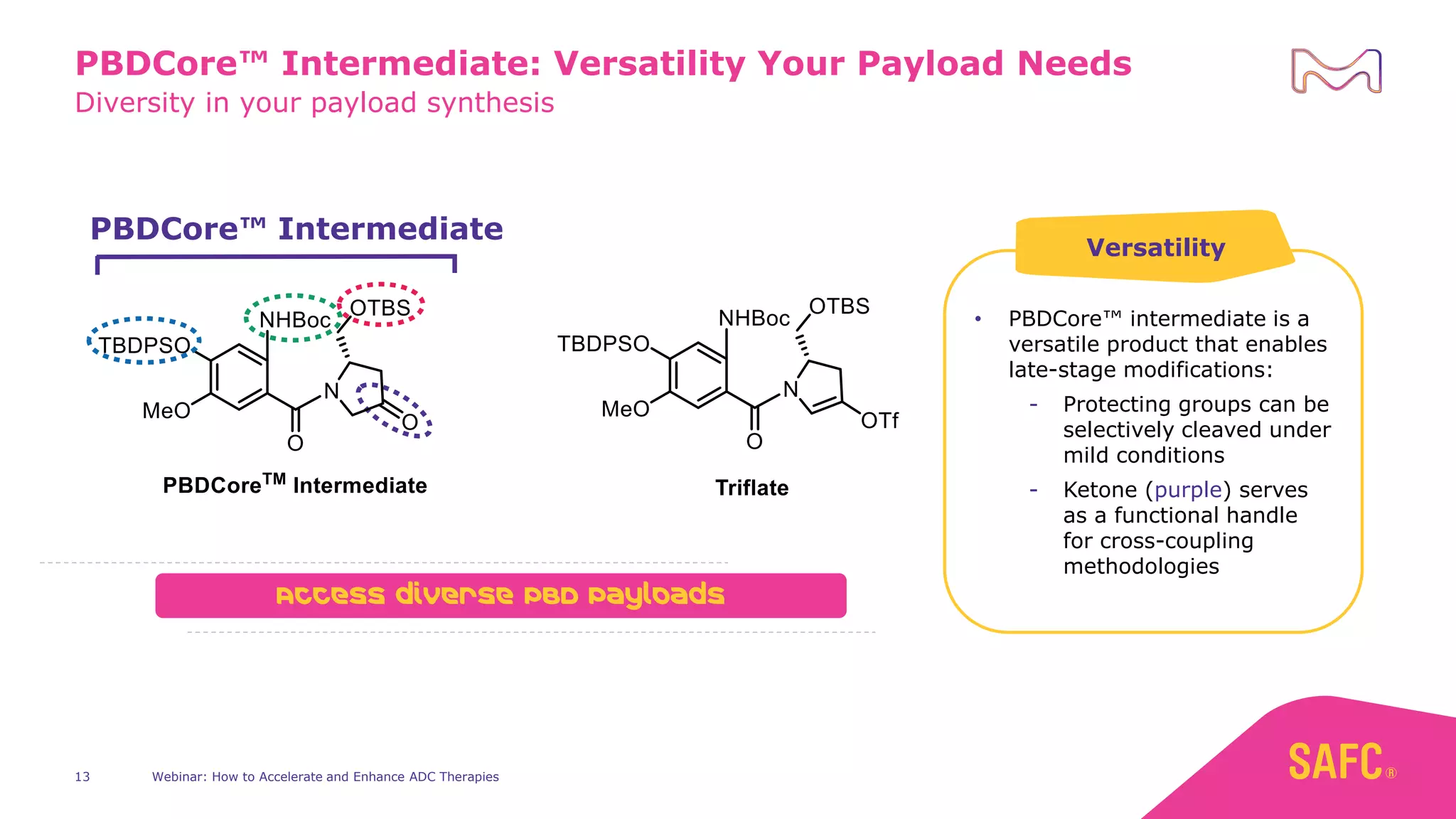
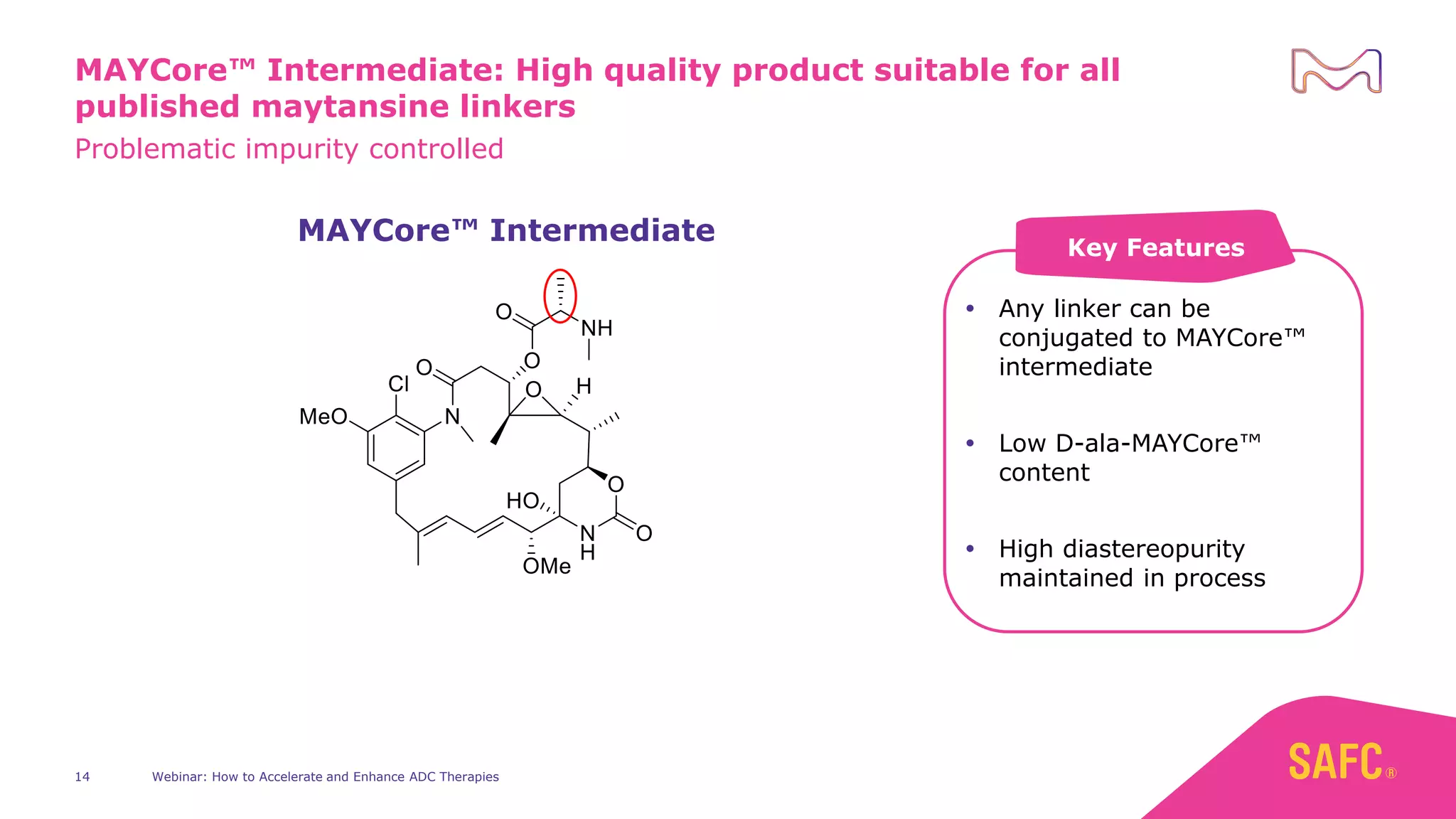
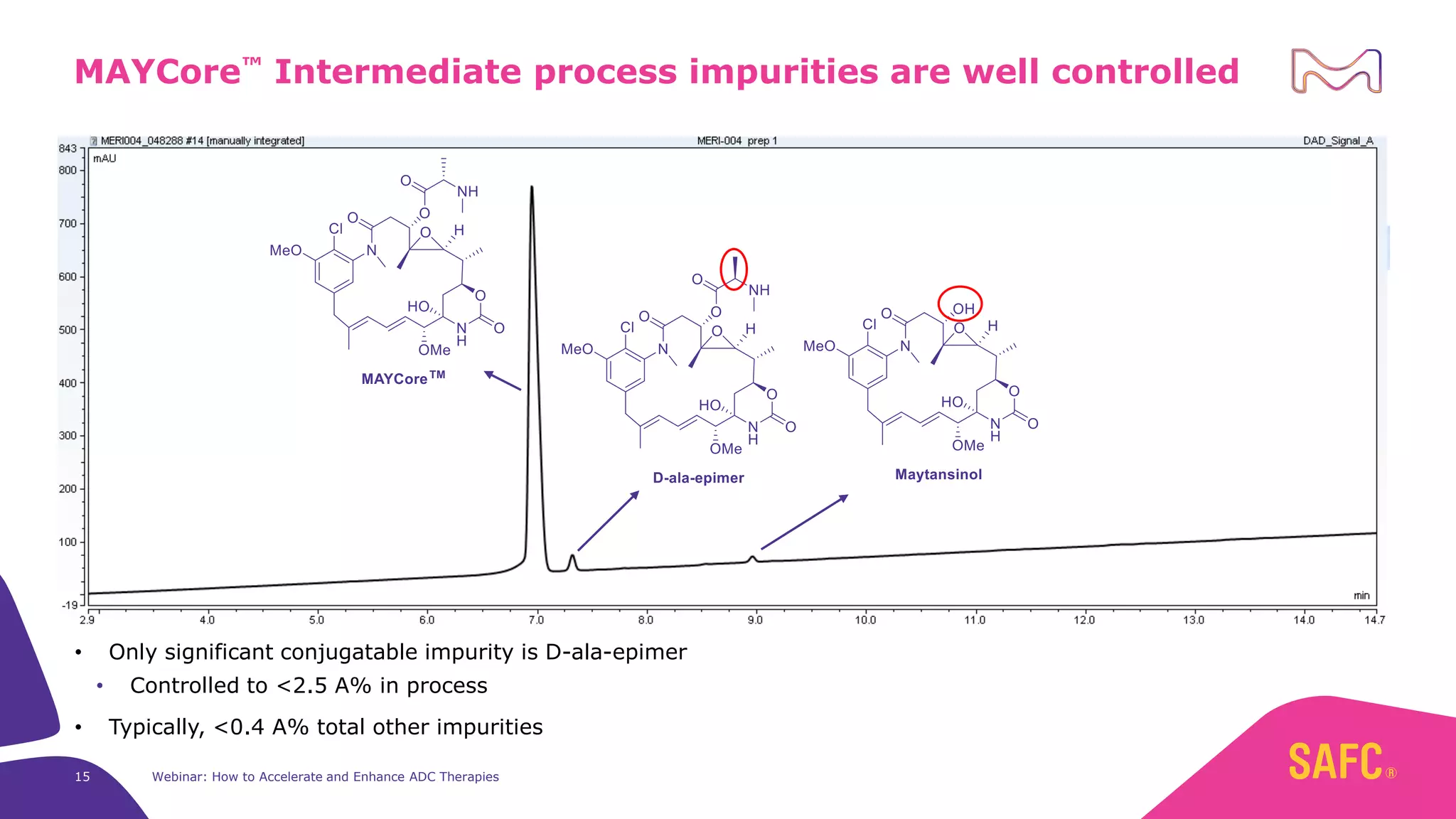
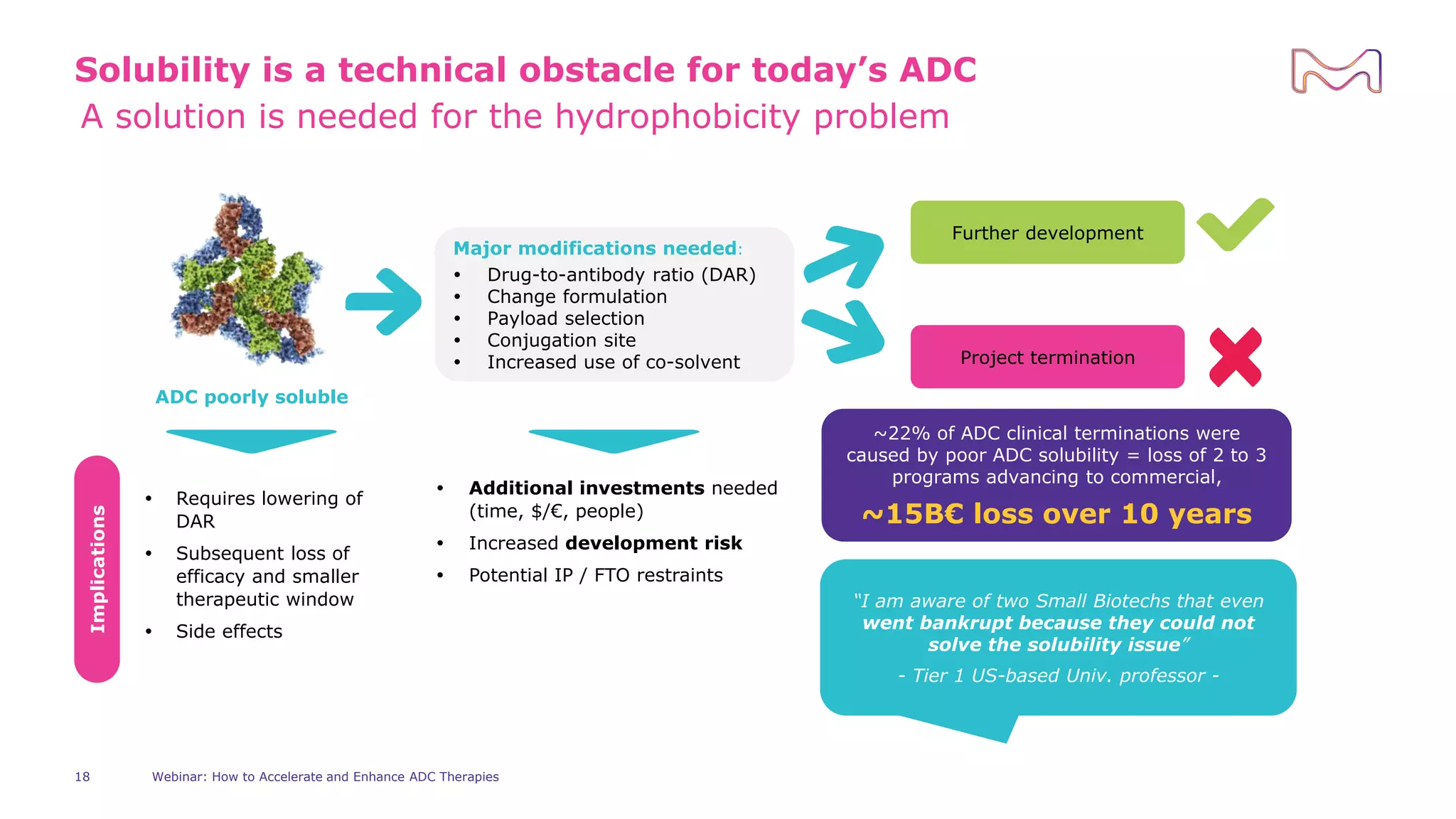
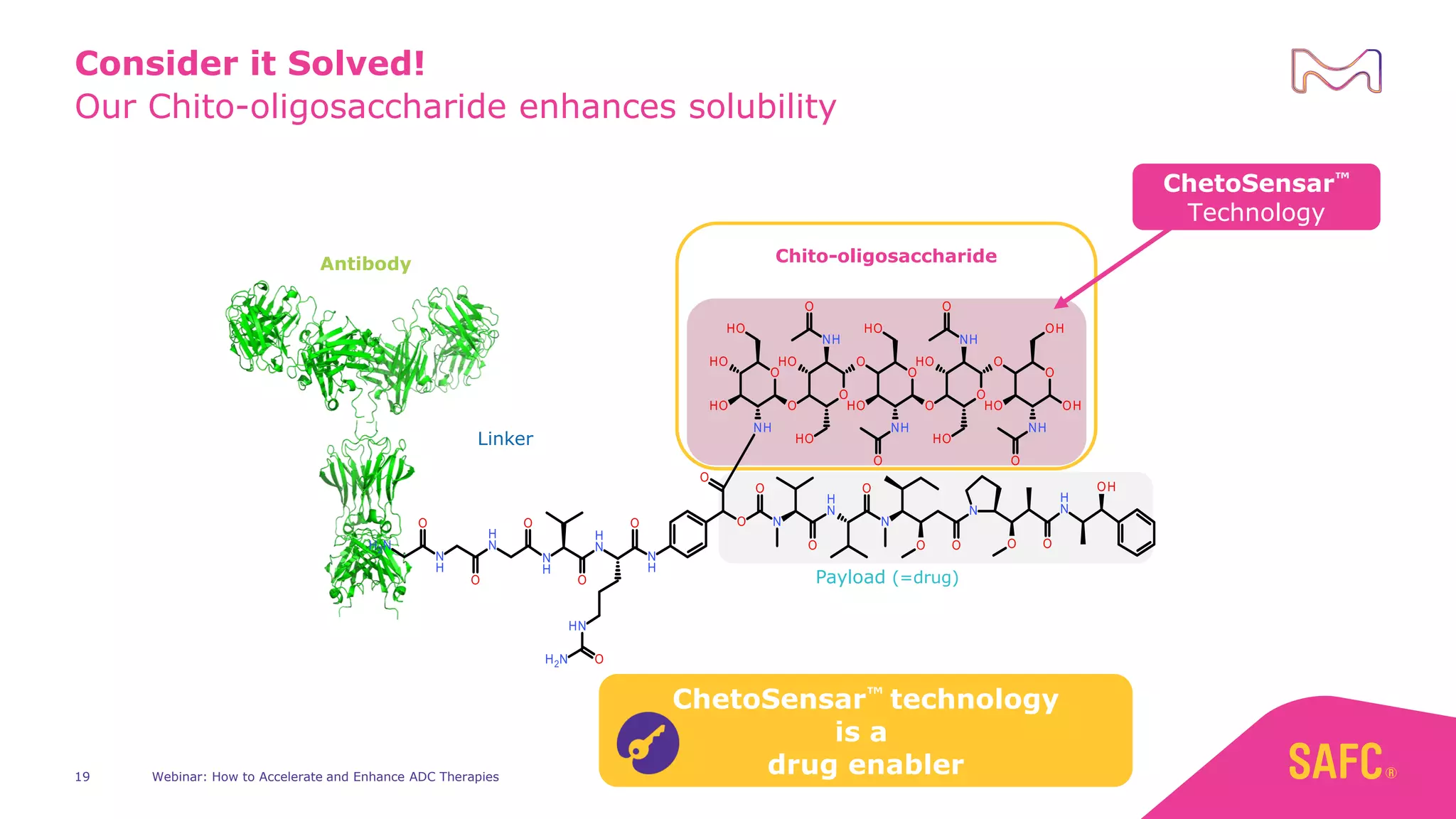
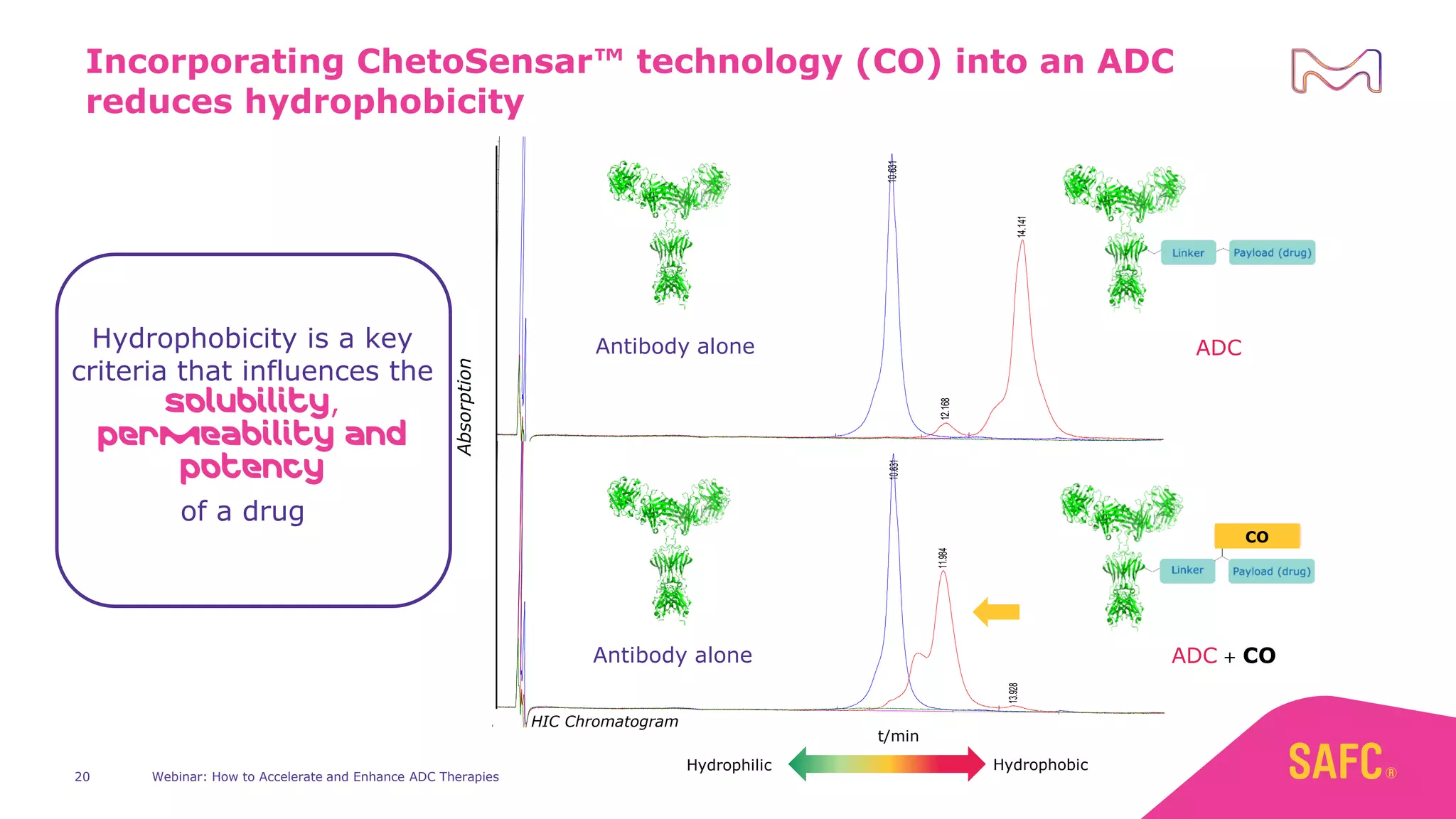
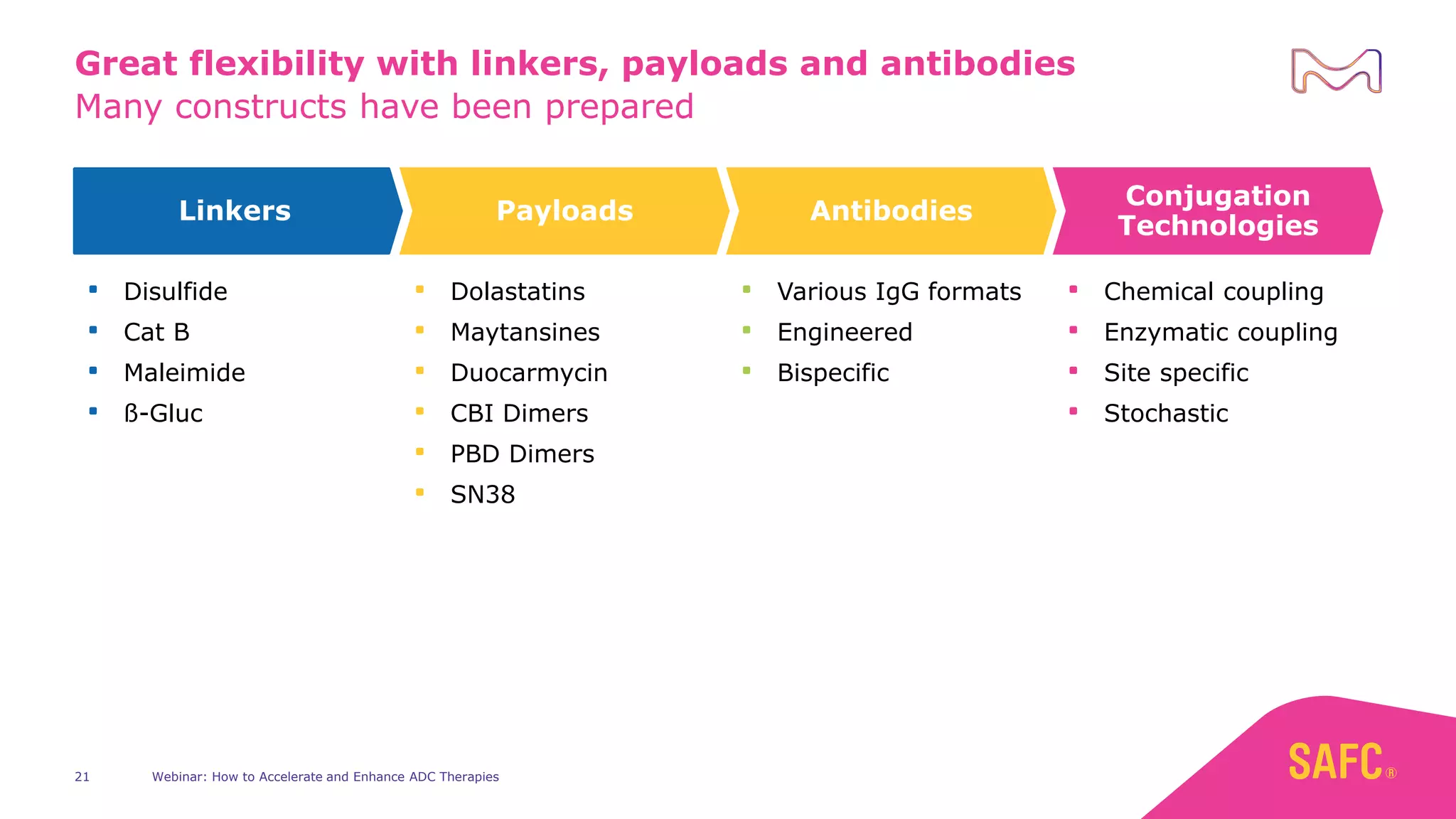
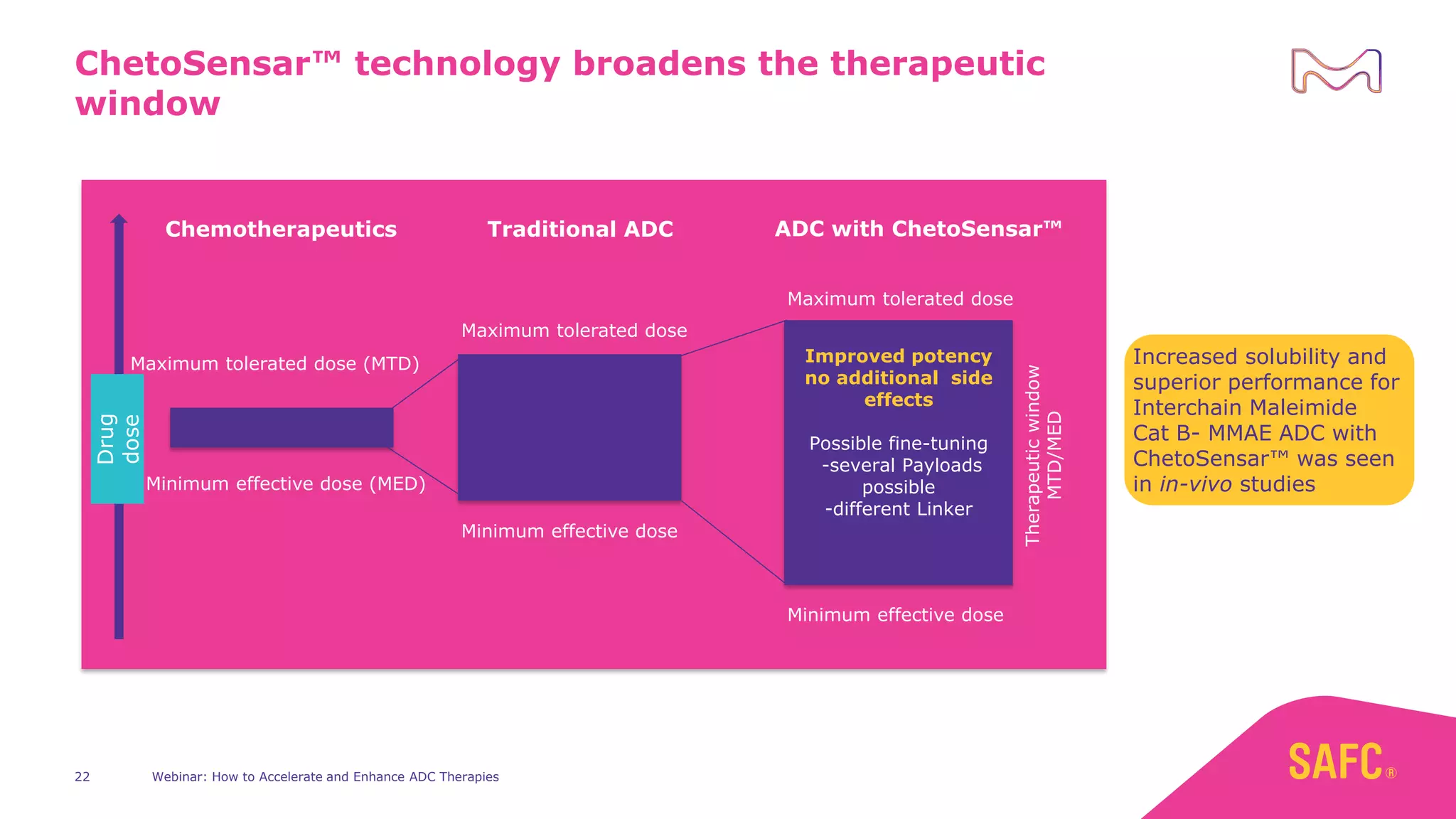
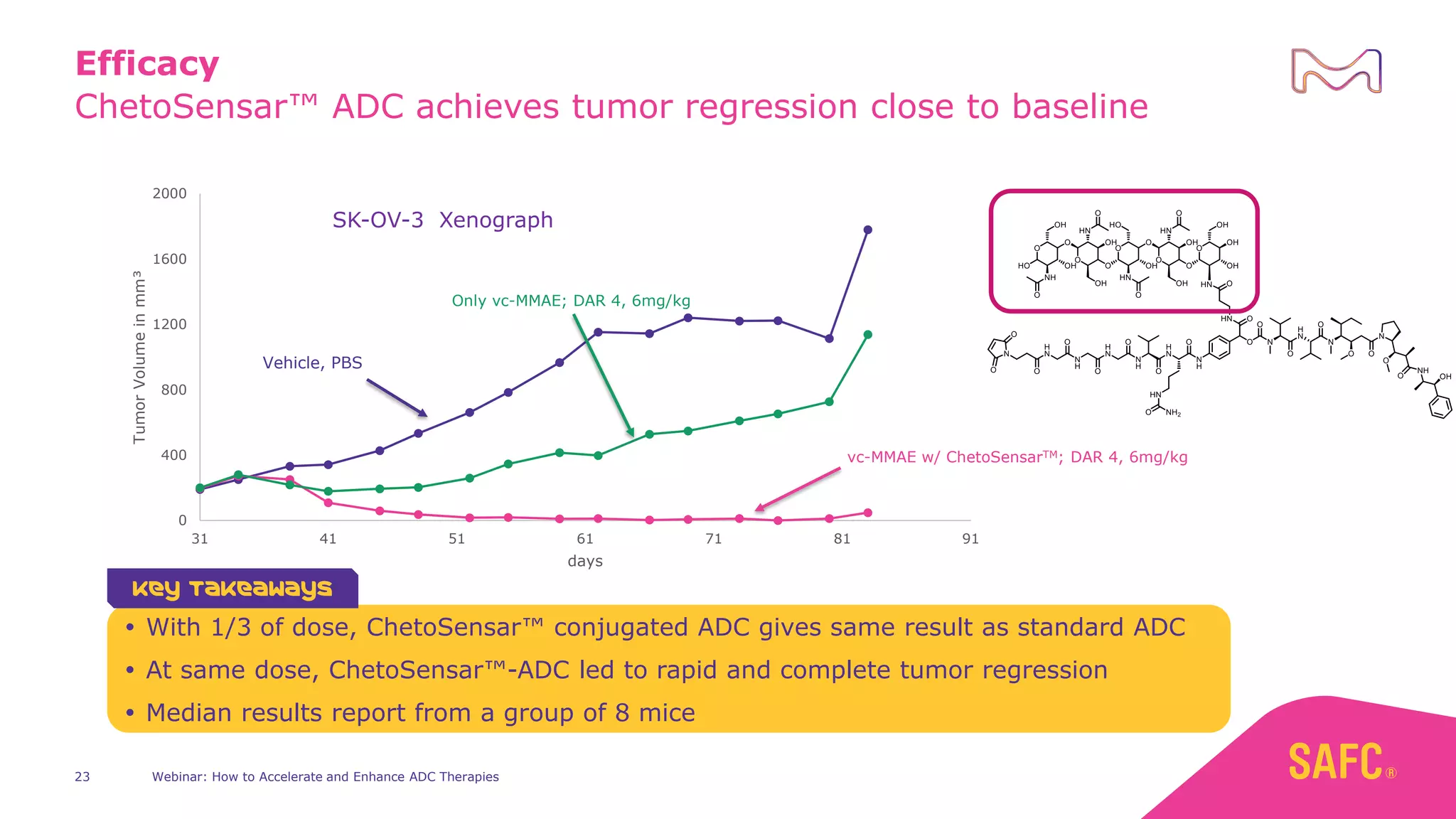
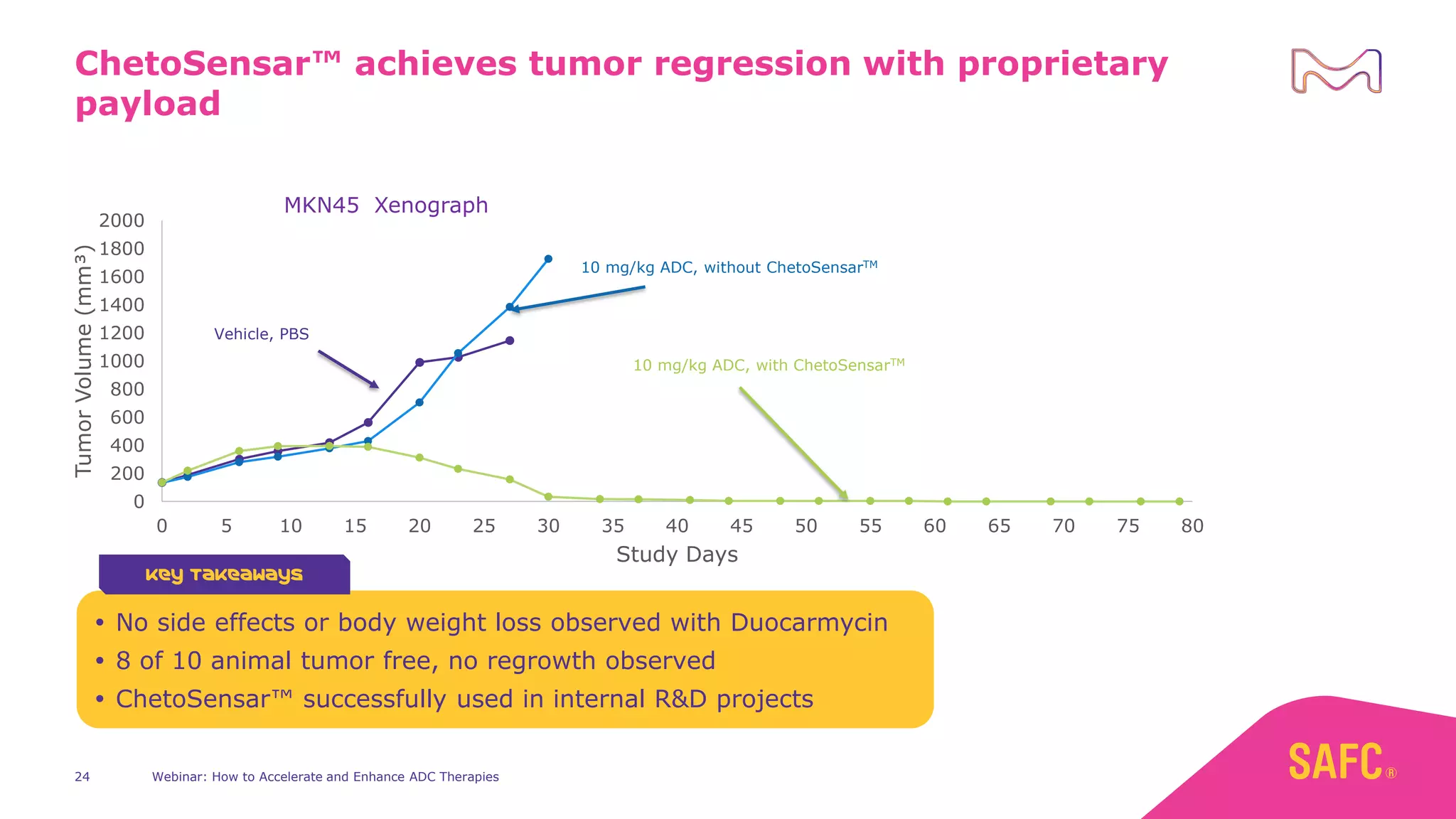
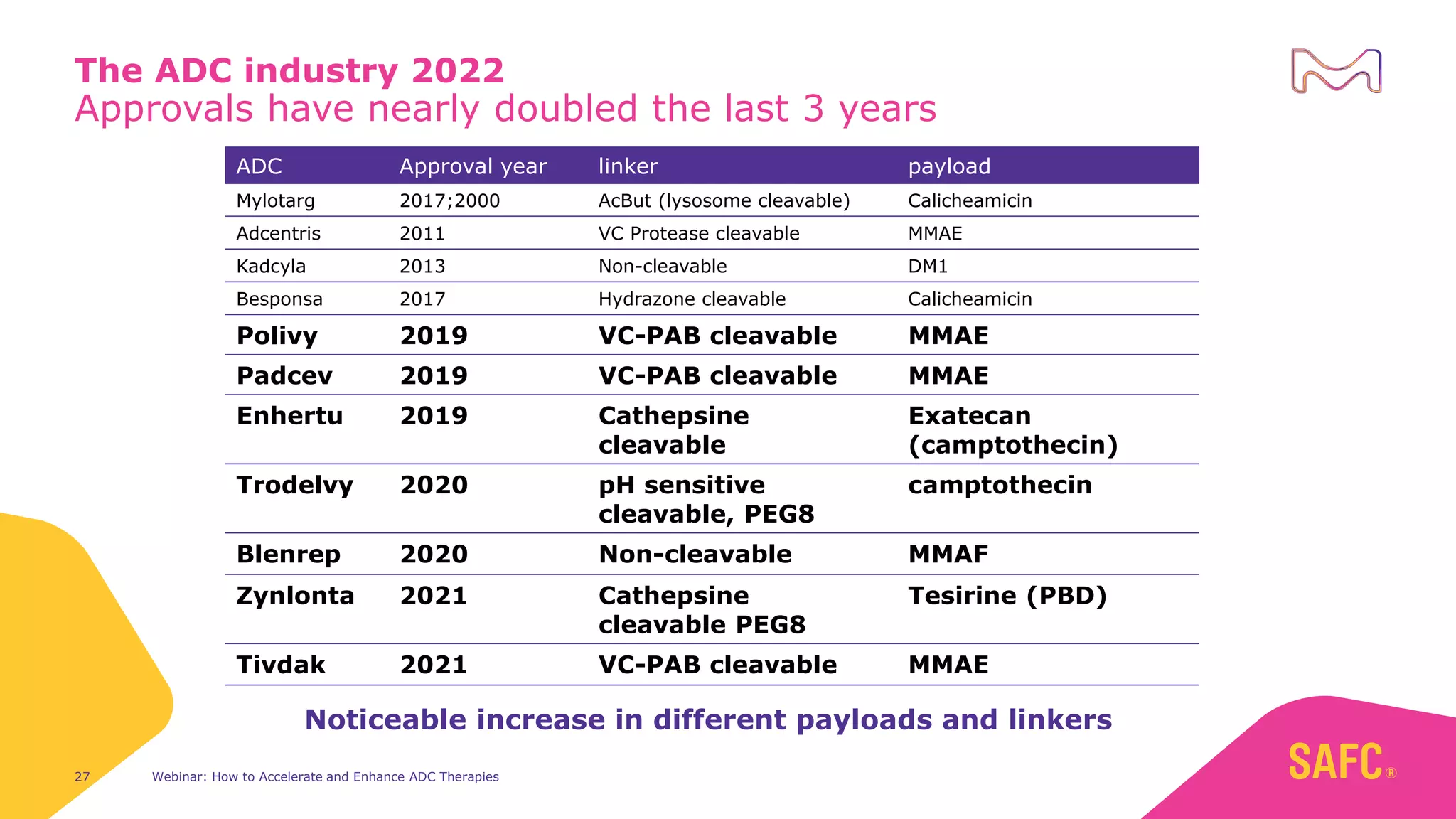
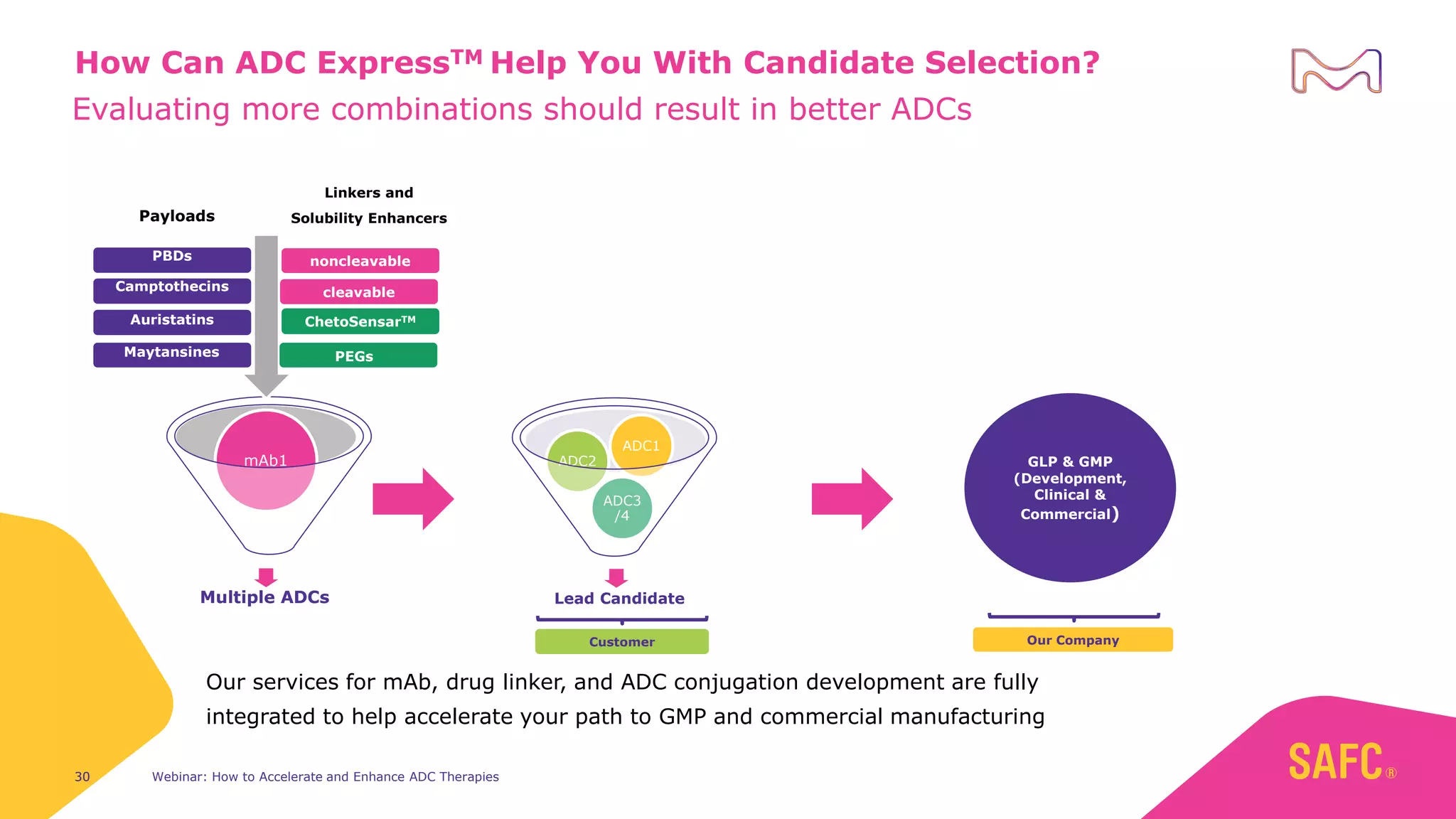
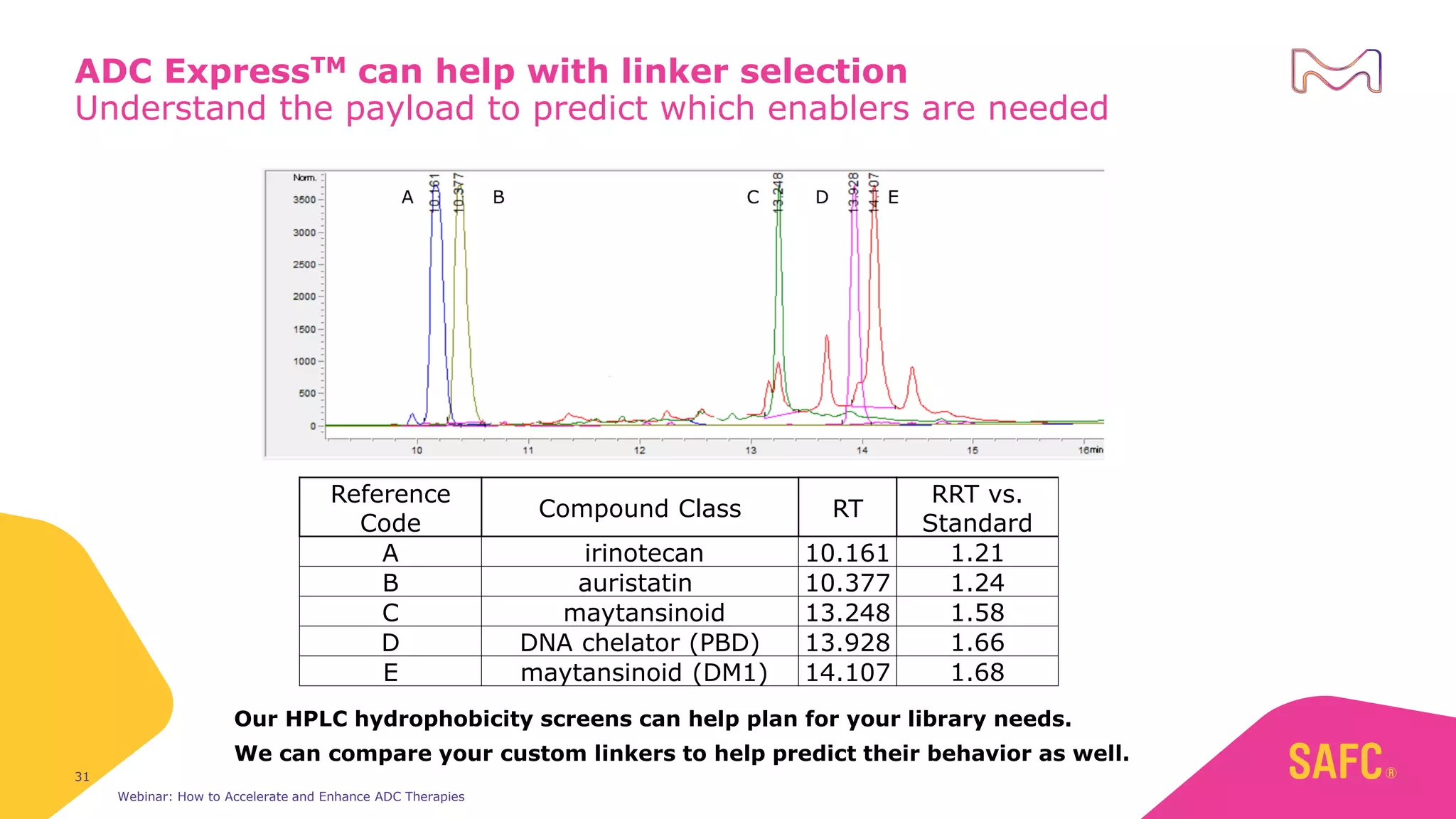
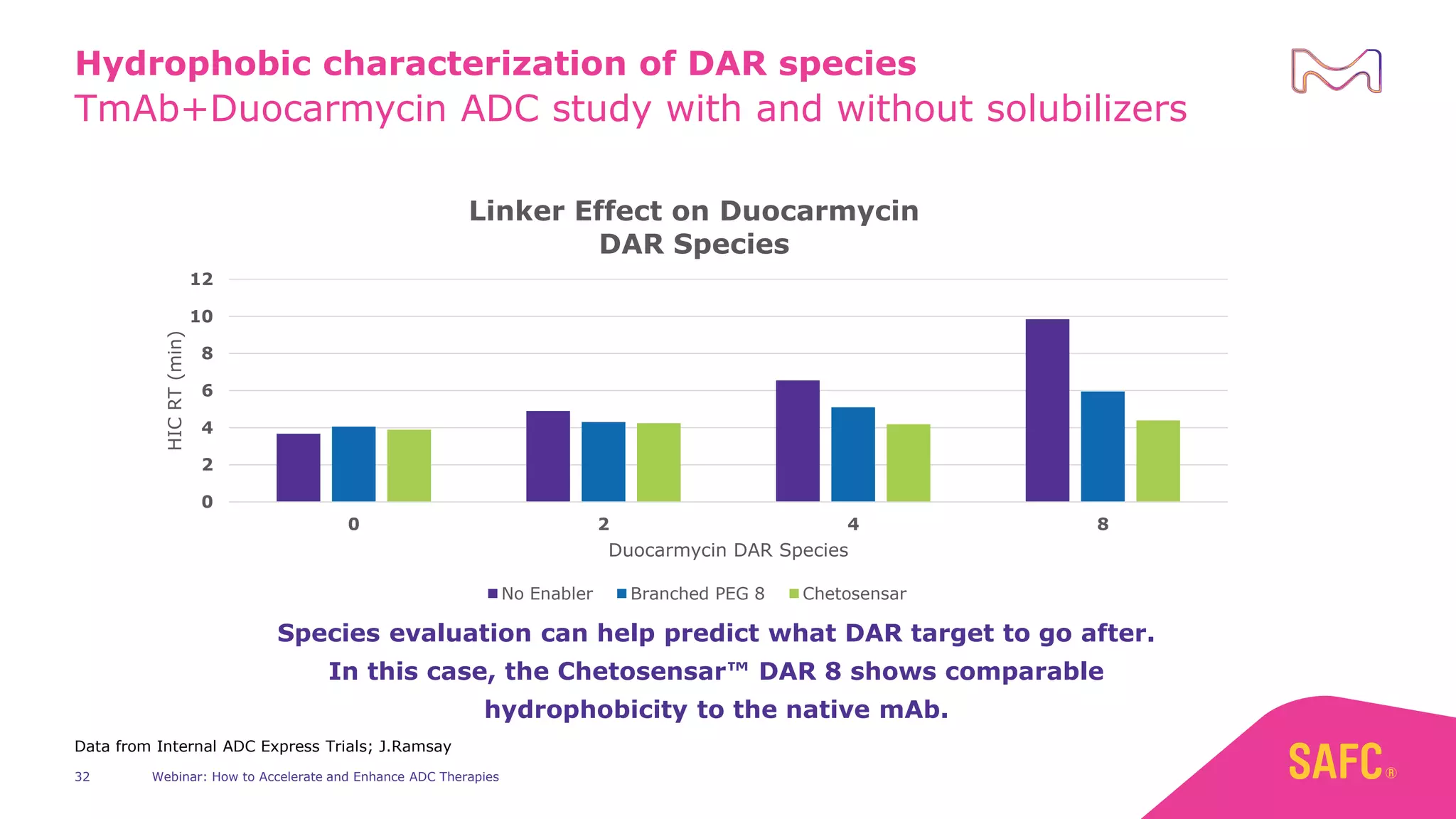
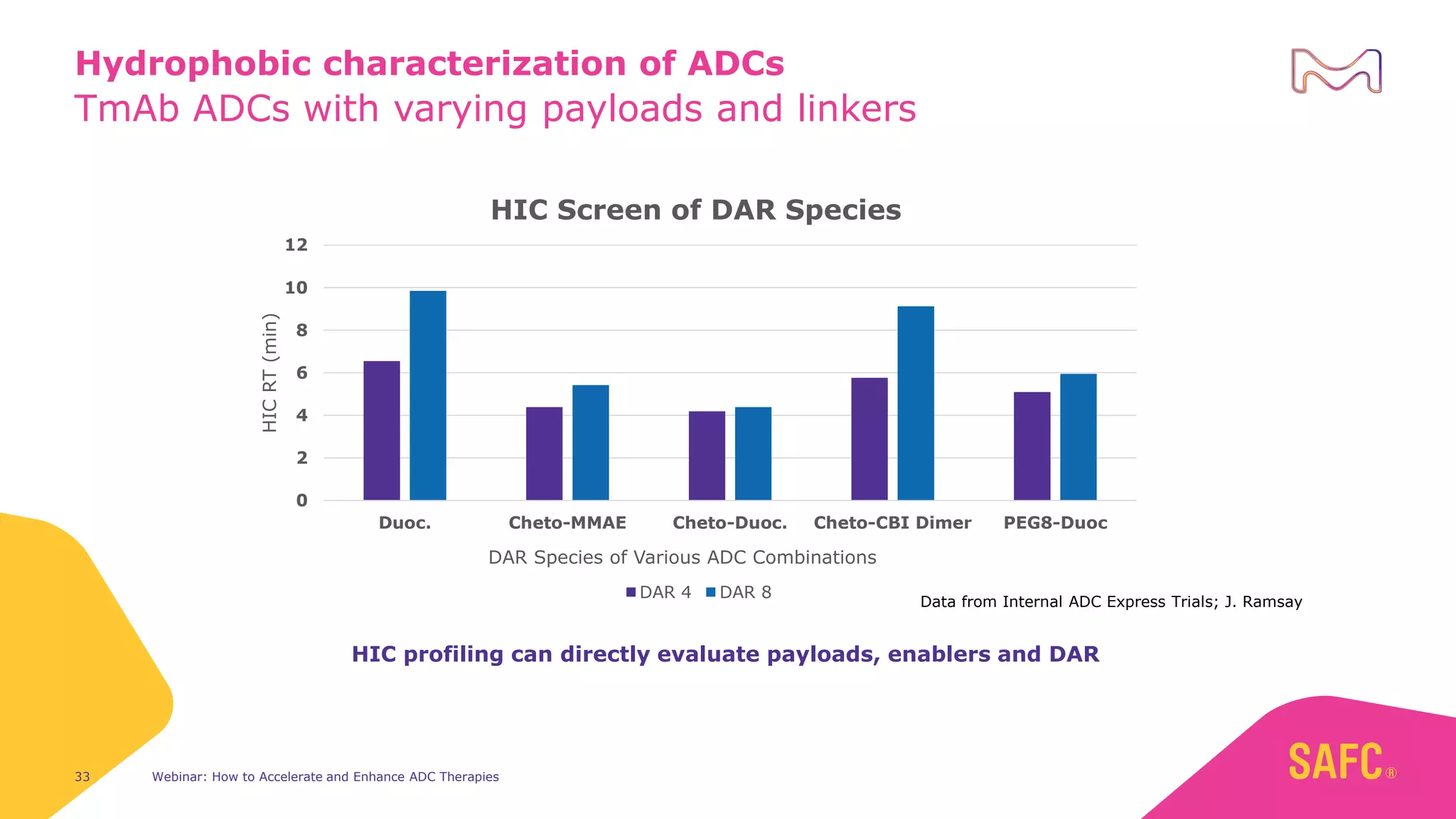
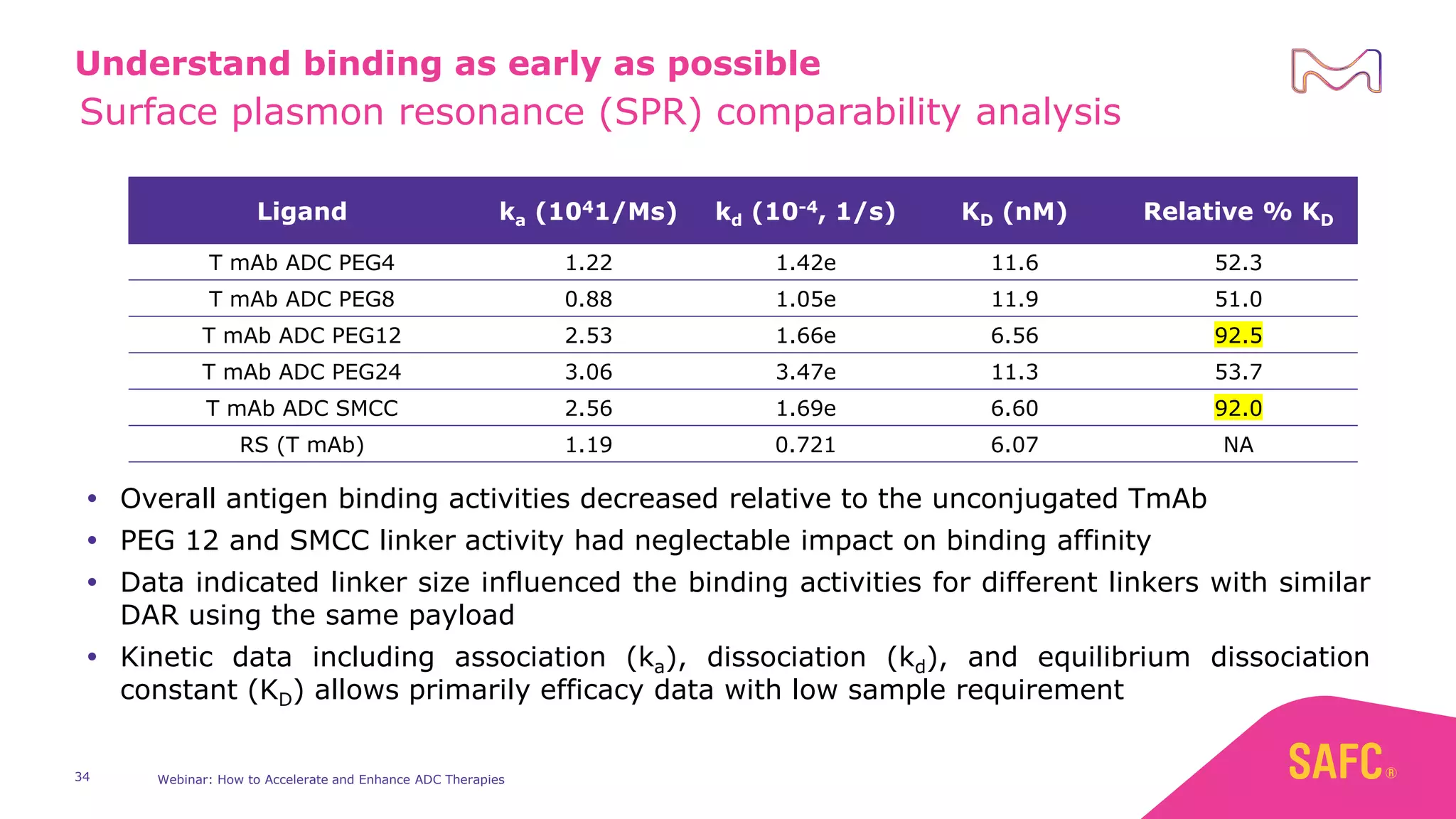
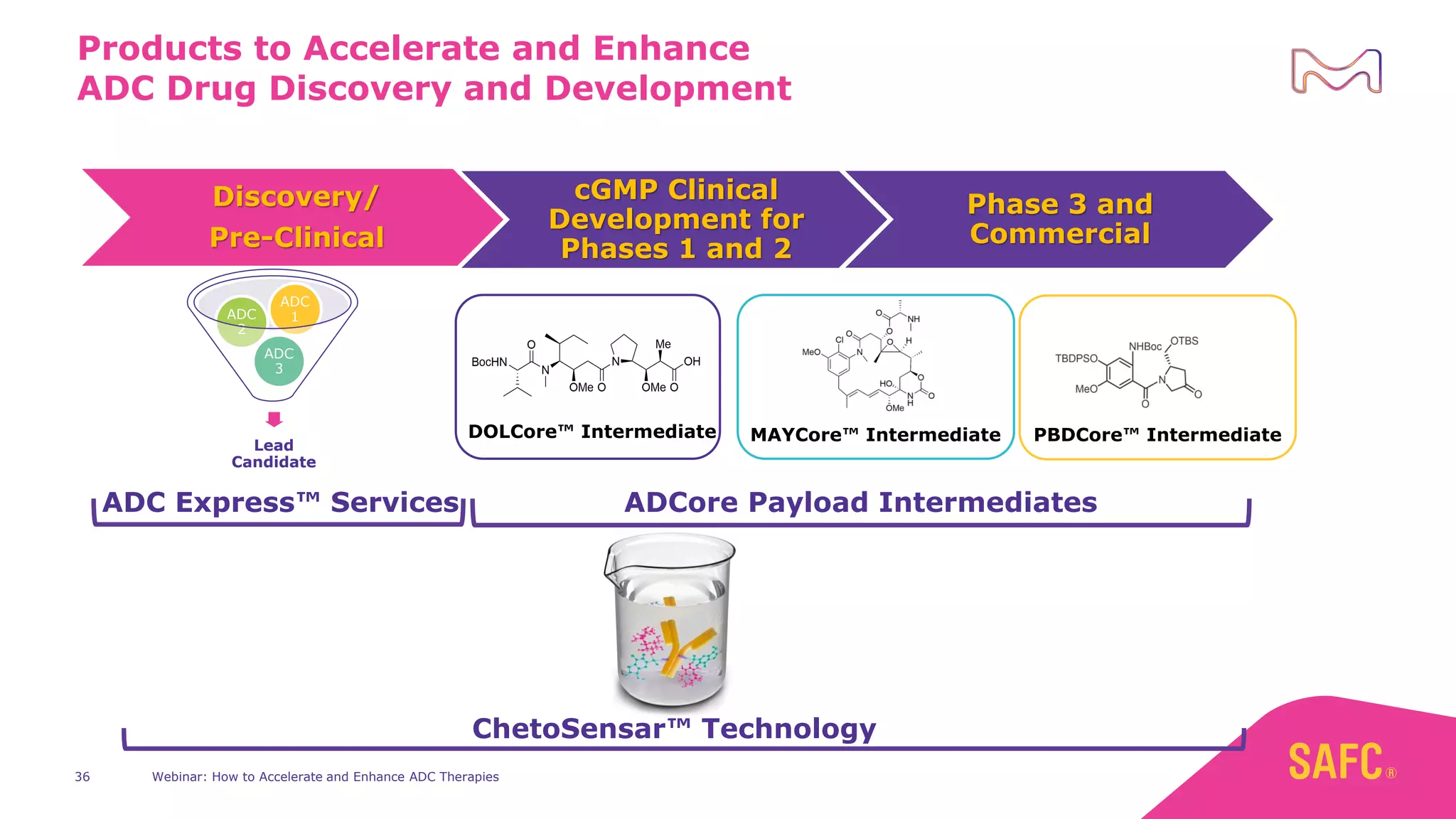
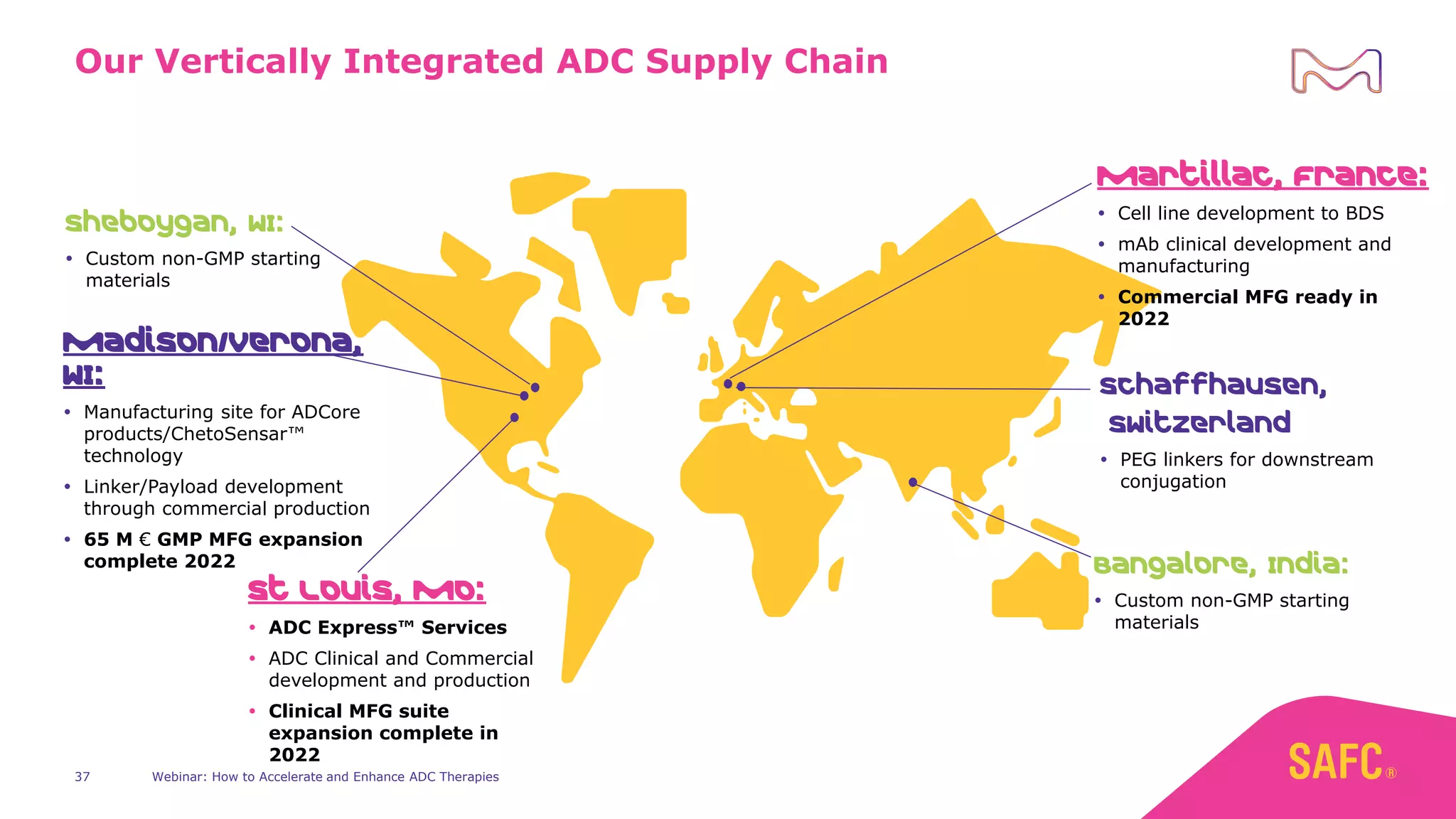
The webinar discusses services from MilliporeSigma to accelerate antibody-drug conjugate (ADC) development through their ADC Express and ADCore product lines. ADC Express provides integrated antibody, linker, payload, and conjugation services to generate multiple ADC candidates for evaluation. The ADCore product line offers intermediates that simplify payload synthesis and accelerate development timelines. ChetoSensar technology incorporates a chito-oligosaccharide to enhance ADC solubility and efficacy.