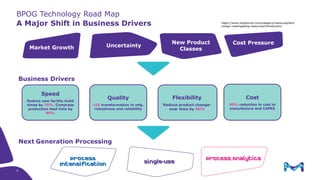
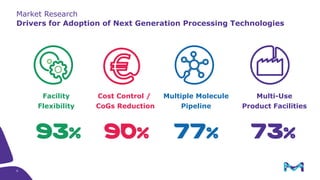
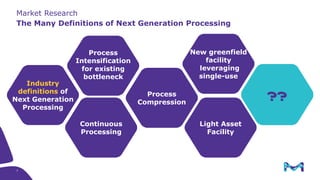
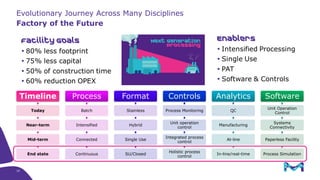
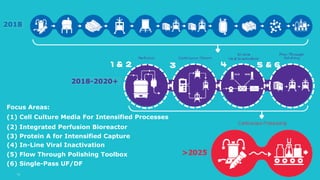
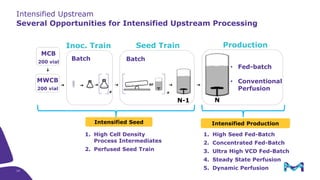
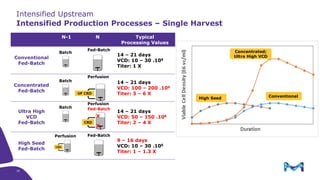
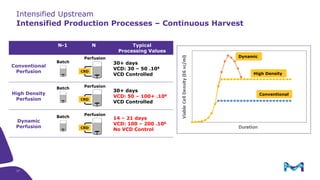
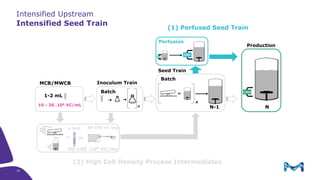
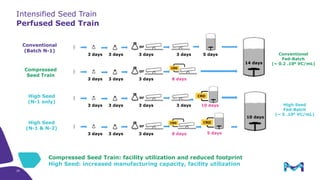
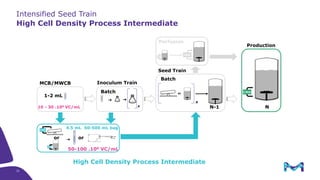
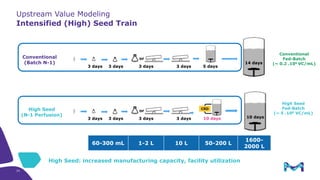
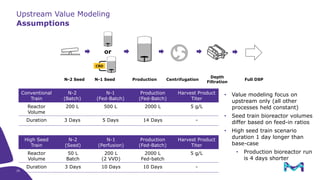
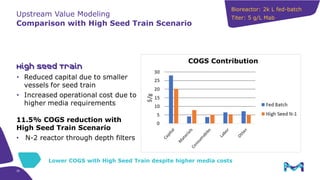
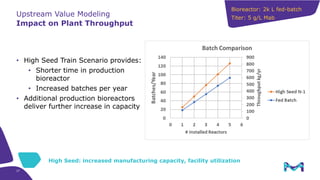
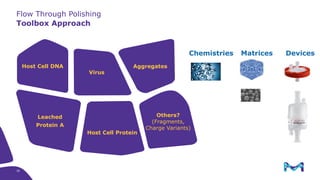
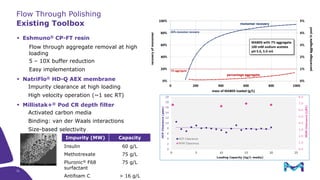
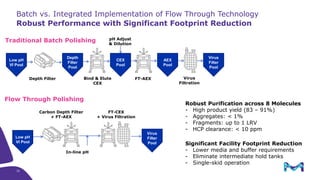
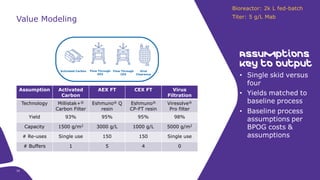
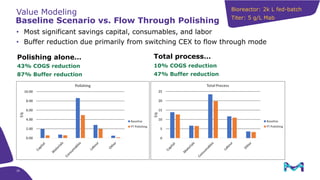
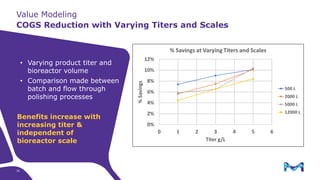
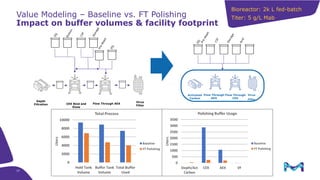
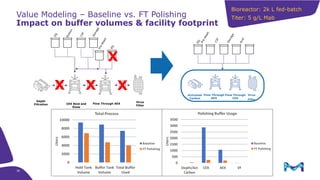
This document discusses the evolution toward continuous monoclonal antibody (mAb) manufacturing using next generation processing technologies. It describes how intensified upstream processes like perfused seed trains and high cell density fed-batch can reduce process times and increase titers. Flow through polishing is also examined as having the potential to reduce buffer volumes by 80-90% and decrease polishing process times by 70%, thereby lowering costs of goods sold by 5-10% according to process modeling. The document advocates for an evolutionary approach combining intensification, connection, and flexibility to deliver higher speed, lower costs and risks in mAb production.