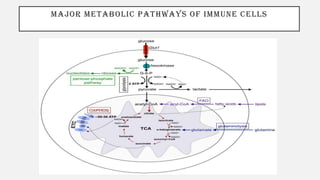
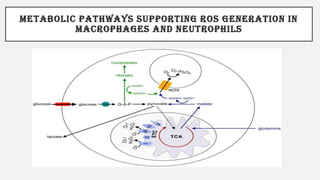
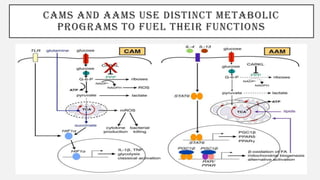
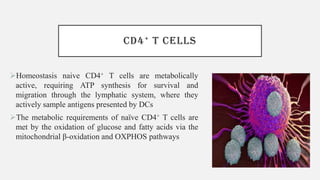
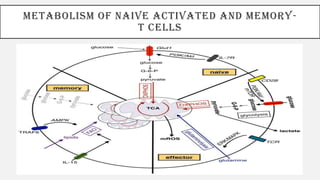
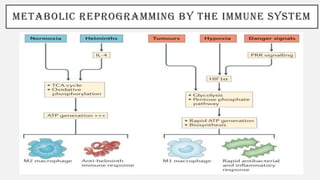
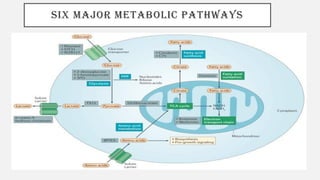
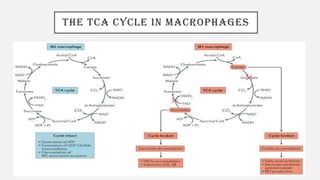
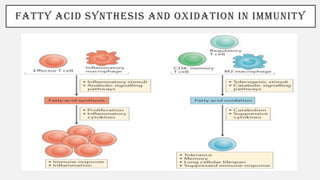
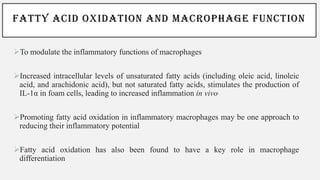
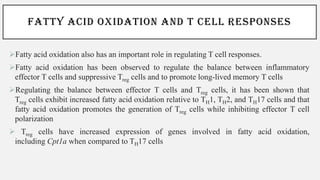
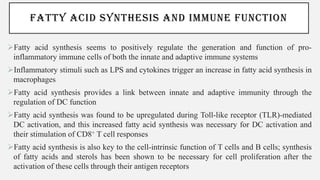
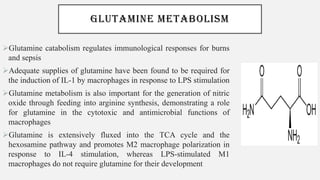
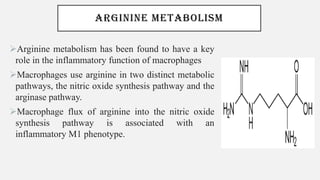
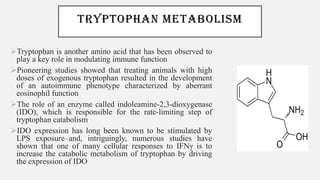
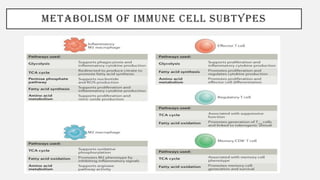
This document discusses immunometabolism, which is the interplay between metabolism and immunology. It describes the two types of immunometabolism - systemic and cellular. Key metabolic pathways in immune cells like glycolysis and fatty acid synthesis are discussed. The document also summarizes how different immune cells like macrophages, neutrophils, mast cells, and dendritic cells regulate their metabolism to support their immune functions through pathways like glycolysis and oxidative phosphorylation.