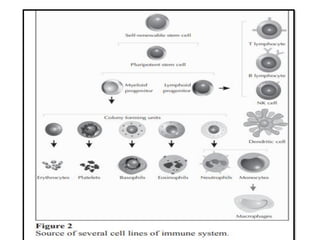
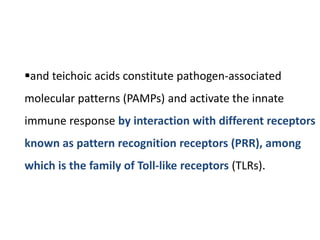
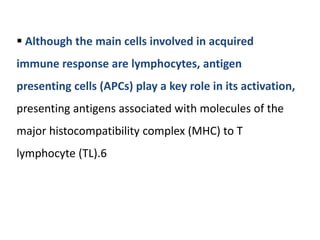
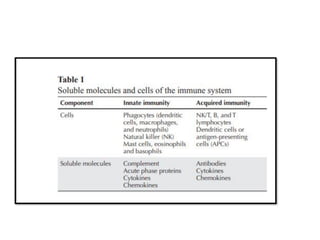
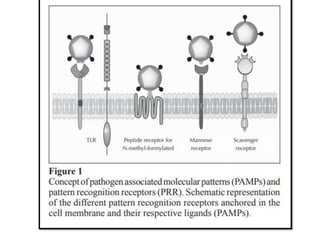
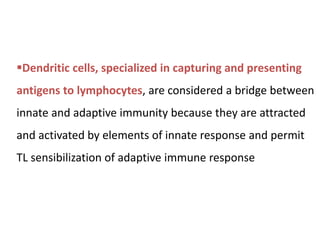
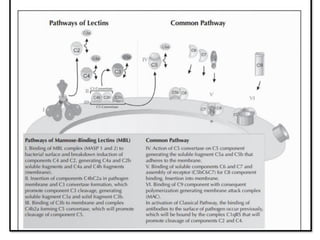
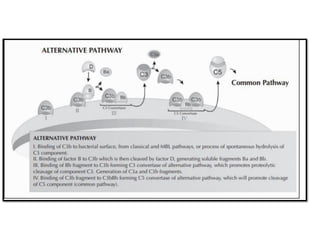
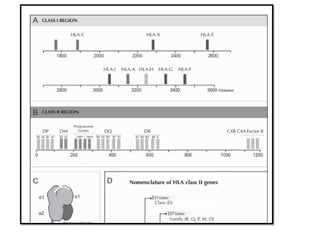
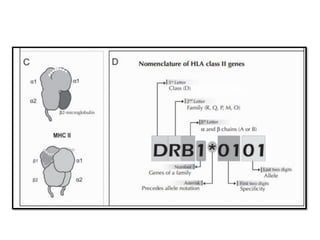
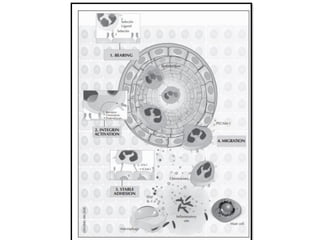
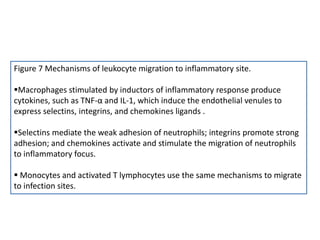
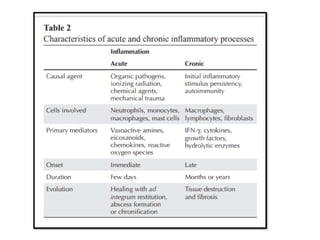
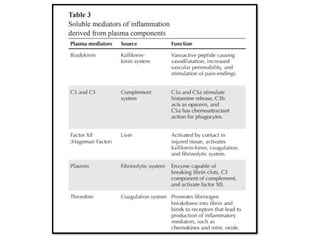
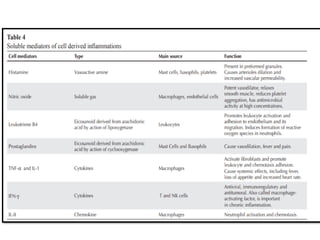
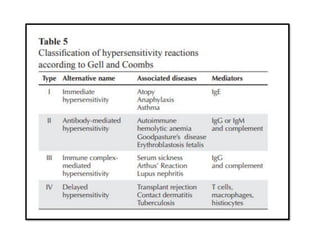
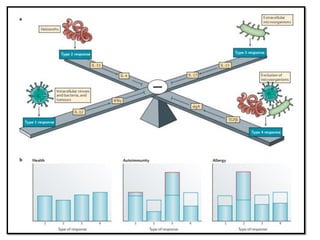
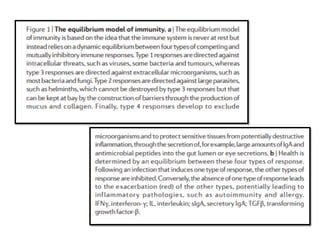
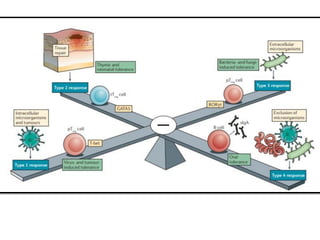
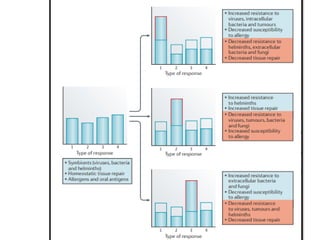
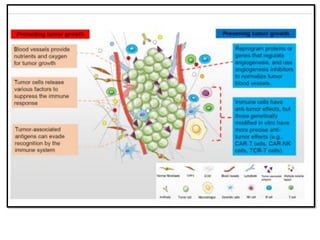
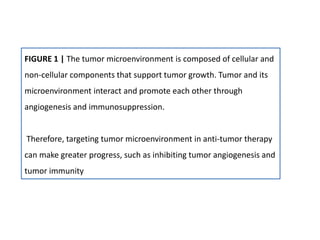
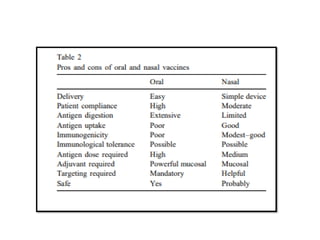
The document covers the functions and mechanisms of the immune system, detailing both innate and adaptive immunity and the roles of various immune cells, such as macrophages, neutrophils, dendritic cells, and natural killer cells. It explains how these cells interact with pathogens and the importance of the complement system and major histocompatibility complex (MHC) in coordinating immune responses. Additionally, it discusses the processes of inflammation and the actions of different immune cells in response to infections and tissue damage.