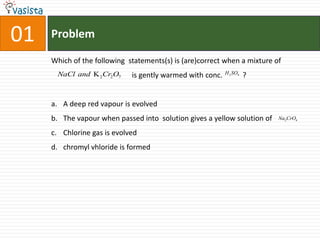
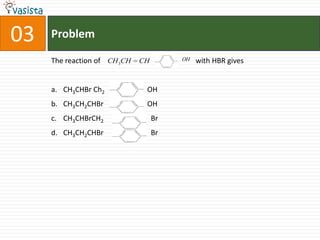
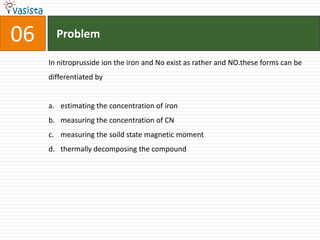
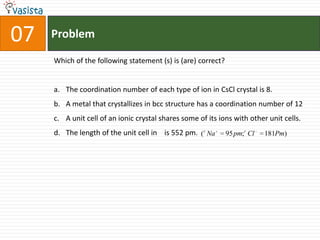
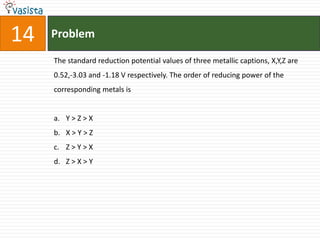
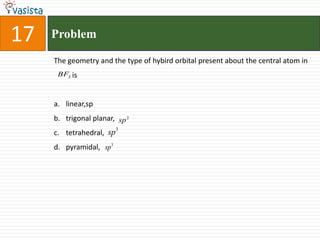
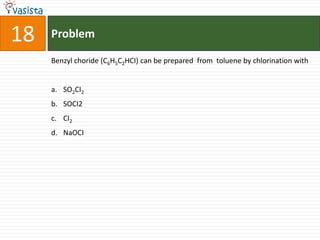
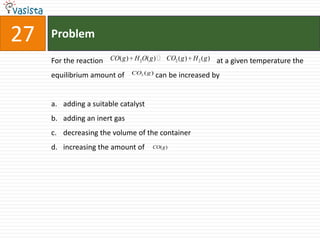
1) The document contains a past paper from 1998 for the IIT JEE chemistry exam with 38 multiple choice and reasoning type questions covering various chemistry concepts.
2) The questions cover topics such as reactions and products (questions 1, 11, 15), electronic configurations (questions 5, 10, 22), isomerism (questions 16, 35), acid-base chemistry (questions 28, 38), and properties of elements and compounds (questions 6, 21, 29, 37).
3) The reasoning questions (29-40) contain an assertion and reason and require evaluating whether the assertion and/or reason are correct and if the reason explains the assertion.