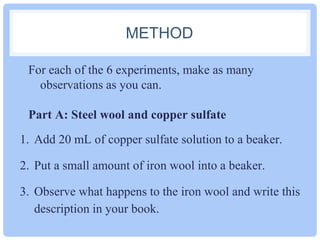
This document outlines six experiments to observe chemical changes. The experiments involve adding iron wool to copper sulfate solution, placing marble chips in hydrochloric acid, mixing sodium sulfate and barium chloride drops, combining bicarbonate of soda and vinegar solutions, adding magnesium to hydrochloric acid, and burning magnesium strips. The goal is to observe signs of chemical reactions occurring and identify common indicators that a chemical reaction has taken place.