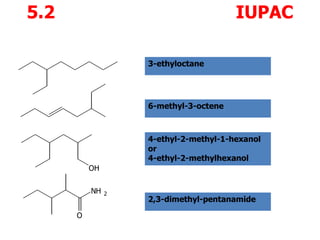
1. IUPAC (International Union of Pure and Applied Chemistry) nomenclature provides systematic names for organic compounds that unambiguously describe their structure.
2. The IUPAC name includes the parent name for the longest carbon chain, prefixes to describe any substituents, and suffixes to indicate functional groups.
3. Examples of IUPAC names are 3-ethyl-2-methylhexane, 1-methylcyclohexene, 3-chlorocyclopentanone, 2-heptene, and 2-hexanol.