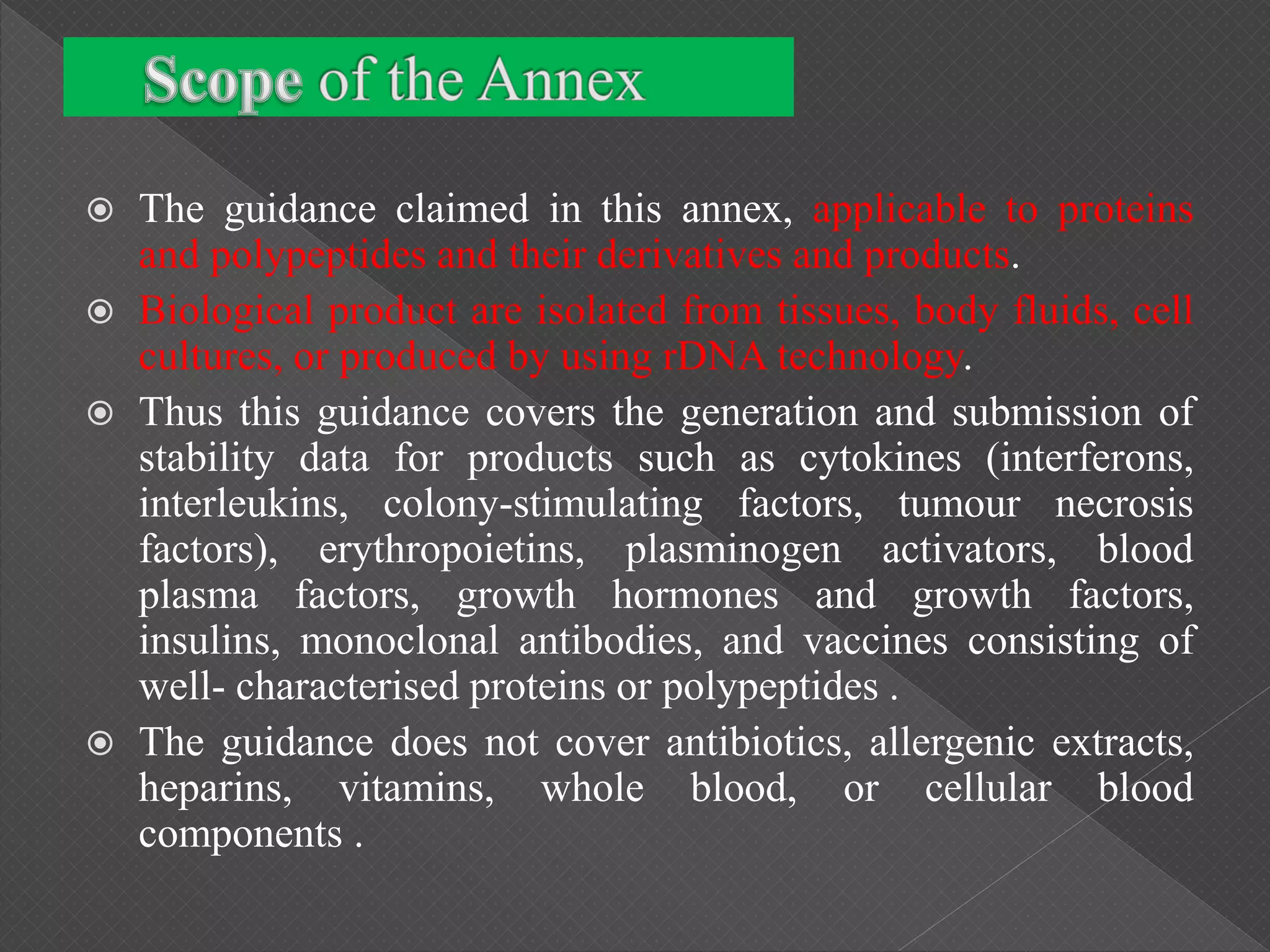
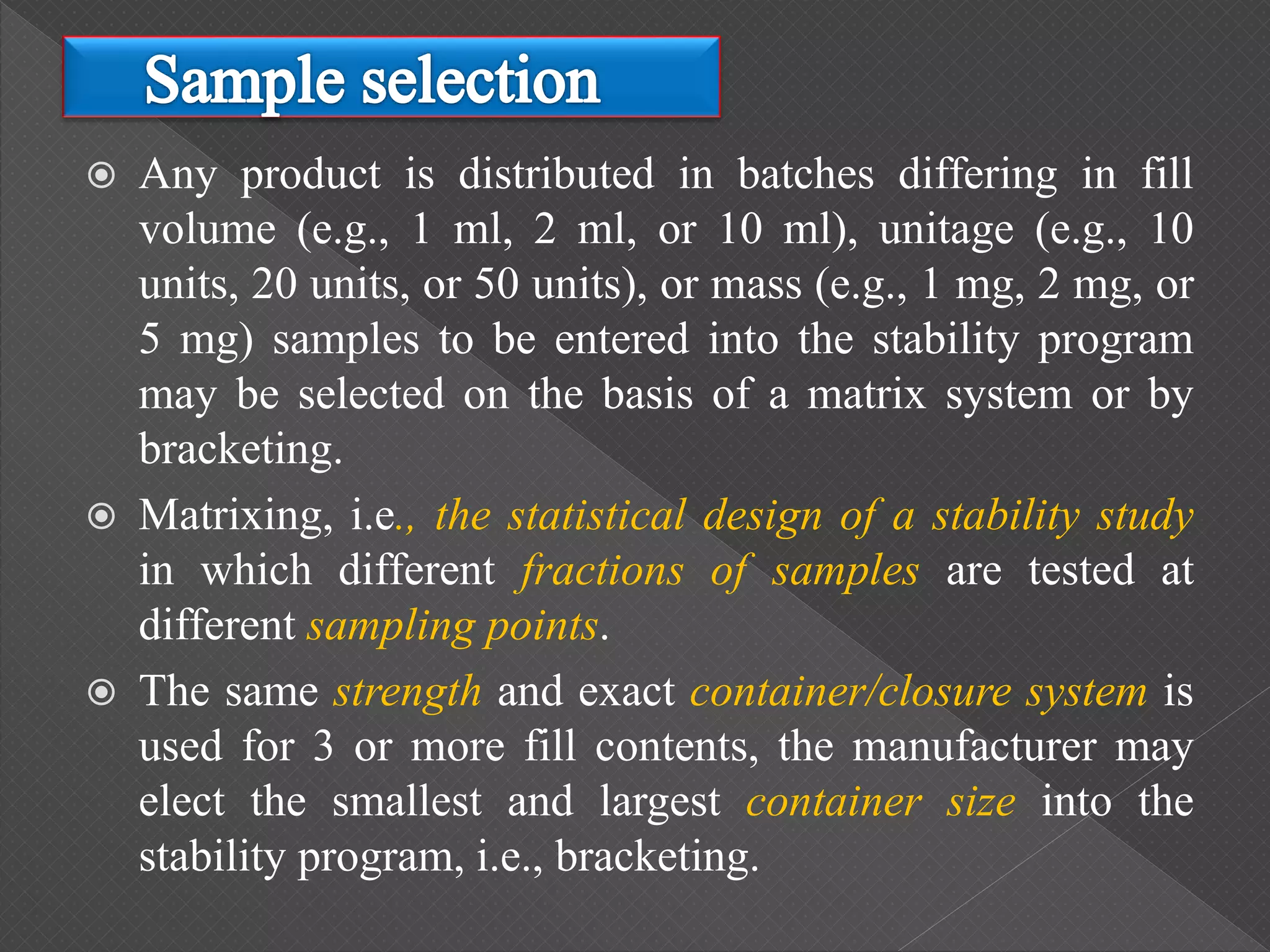
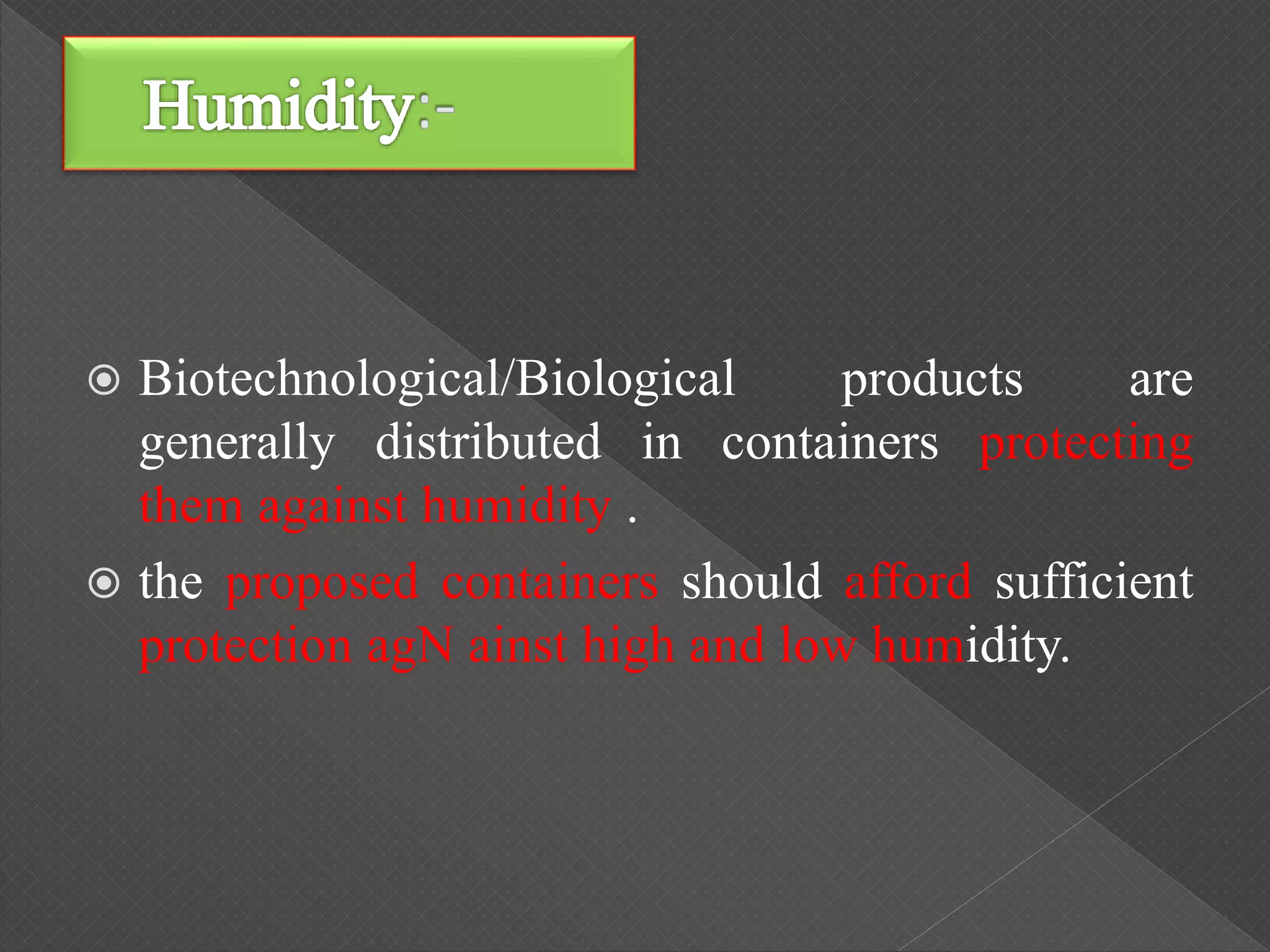
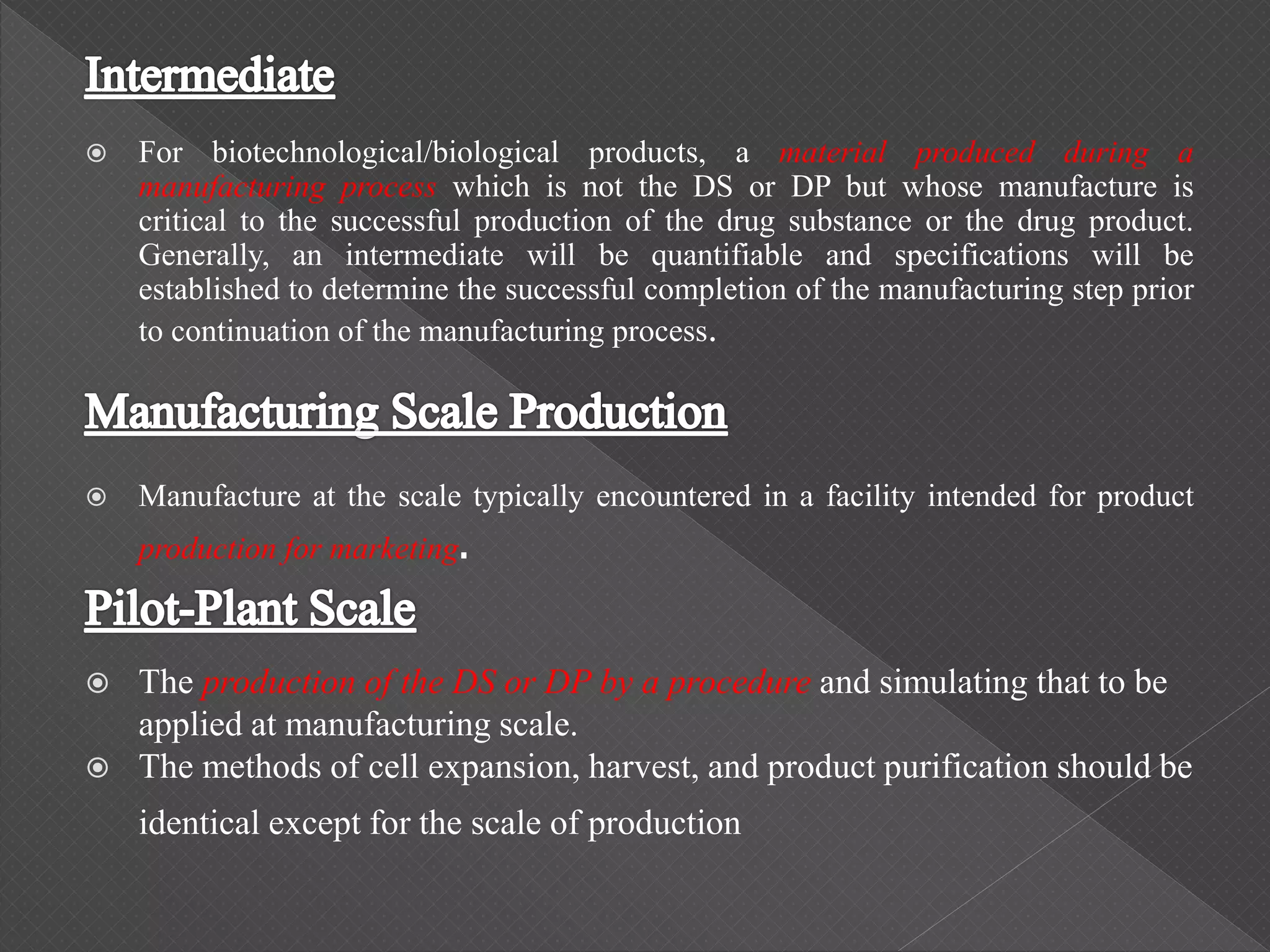
The ICH Q5C guideline, introduced in 1995, delineates stability testing requirements for biological products compared to small molecules, outlining the necessity of real-time and real-condition stability studies. It specifies stability data submission for various biological drugs, such as proteins and polypeptides, with clear criteria on batch selection, analytical methodologies, and product characteristics. The guidance aims to ensure that all biotechnological products maintain safety, purity, and potency throughout their proposed shelf life under defined storage conditions.