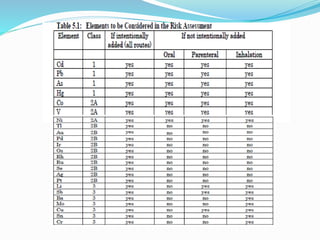
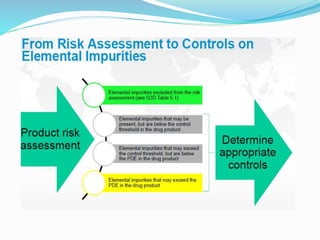
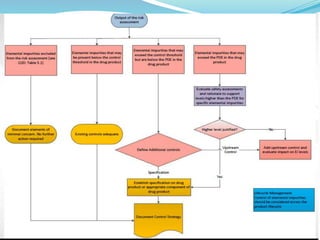
The document discusses elemental impurities that may be present in drug substances and products. It classifies elemental impurities into three classes based on their toxicity and likelihood of occurrence. Class 1 includes highly toxic elements like arsenic, cadmium, mercury and lead that require evaluation. Class 2 elements are route-dependent toxins divided into 2A (cobalt, nickel and vanadium) and 2B (silver, gold, etc.) based on probability of occurrence. Class 3 elements like barium have relatively low oral toxicity but may require consideration for inhalation/parenteral routes. The document provides details on the classification and control of each class of elemental impurities.