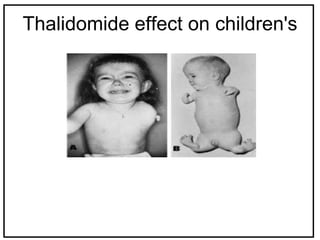
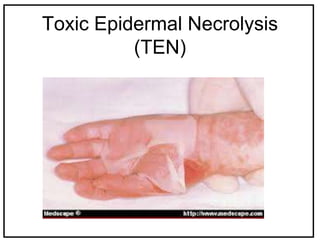
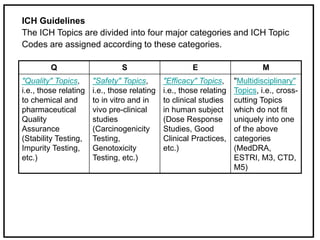
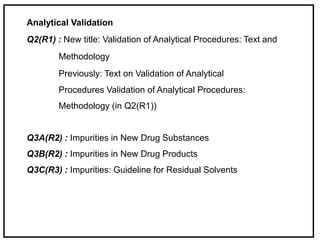
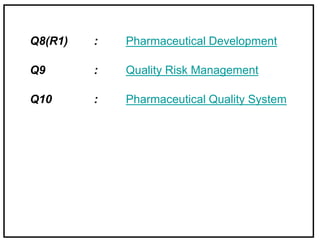
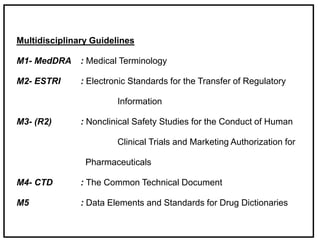
The International Conference on Harmonization (ICH) is a joint regulatory/industry initiative involving drug regulatory authorities and pharmaceutical industry in the European Union, Japan, and the United States. The goal of ICH is to harmonize technical requirements for pharmaceutical product registration among these three regions to ensure safety and efficacy and allow for more efficient drug development. ICH develops harmonized guidelines covering non-clinical, clinical, and quality topics through expert working groups with representatives from regulatory agencies and industry.