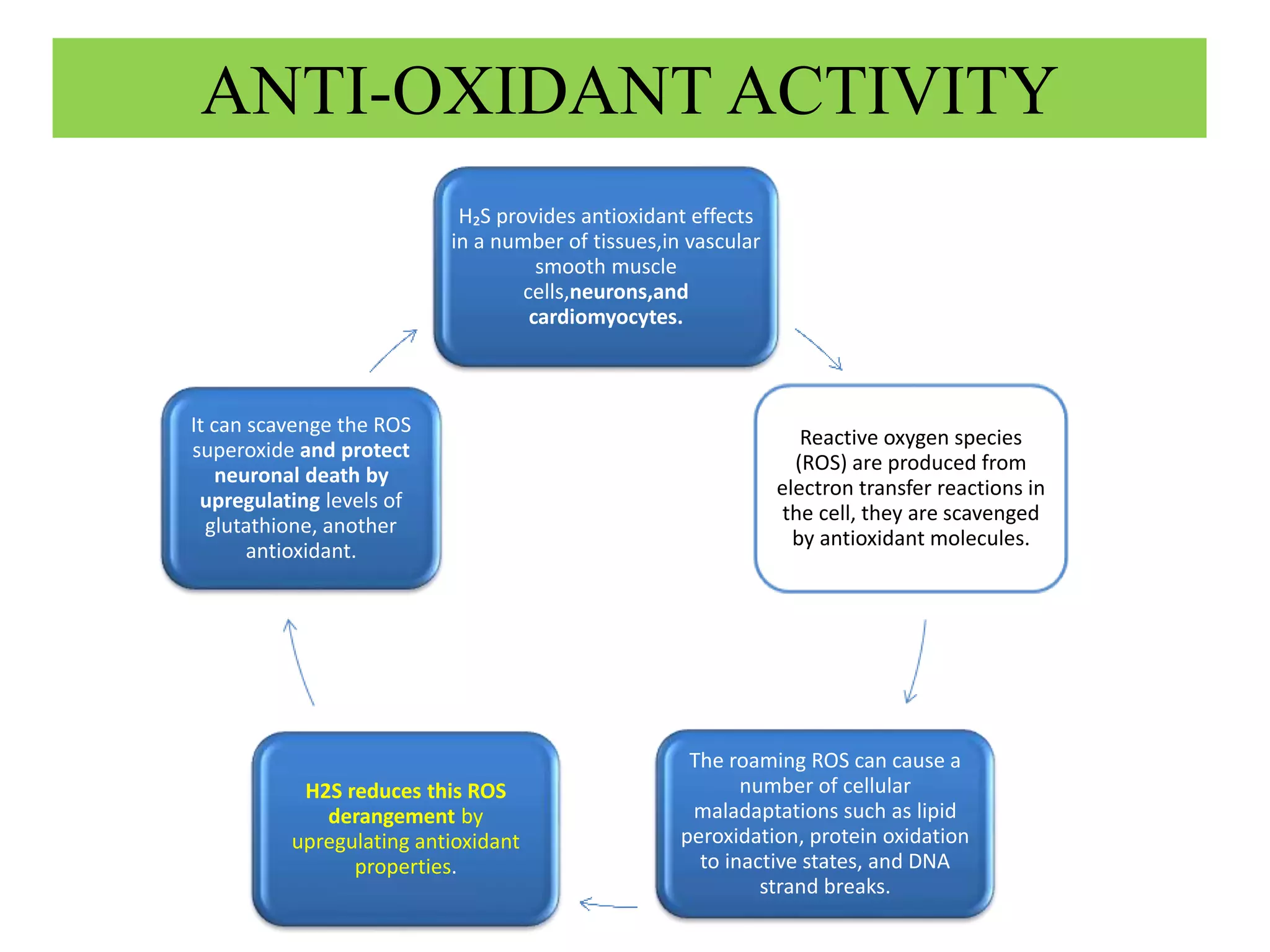
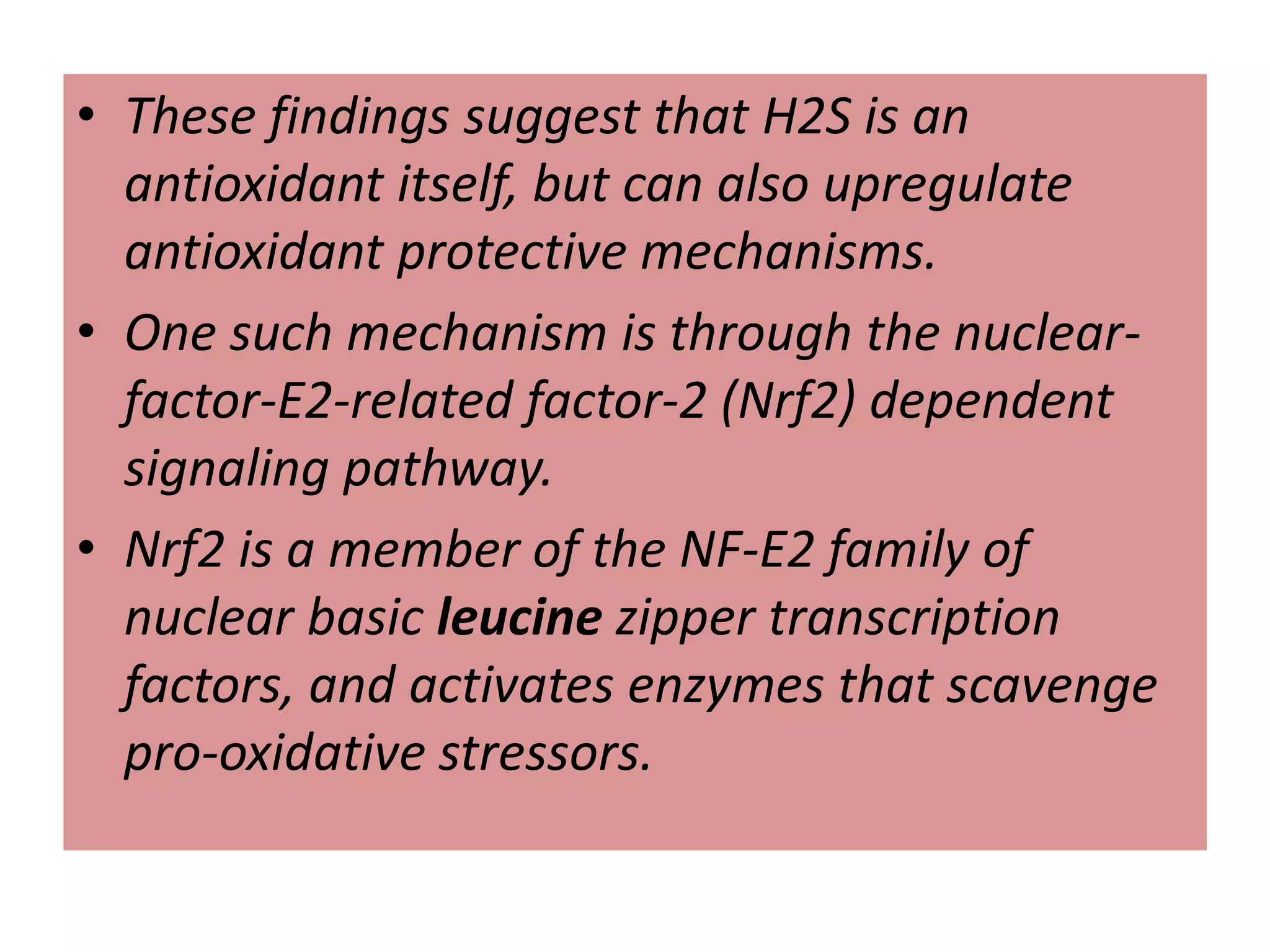
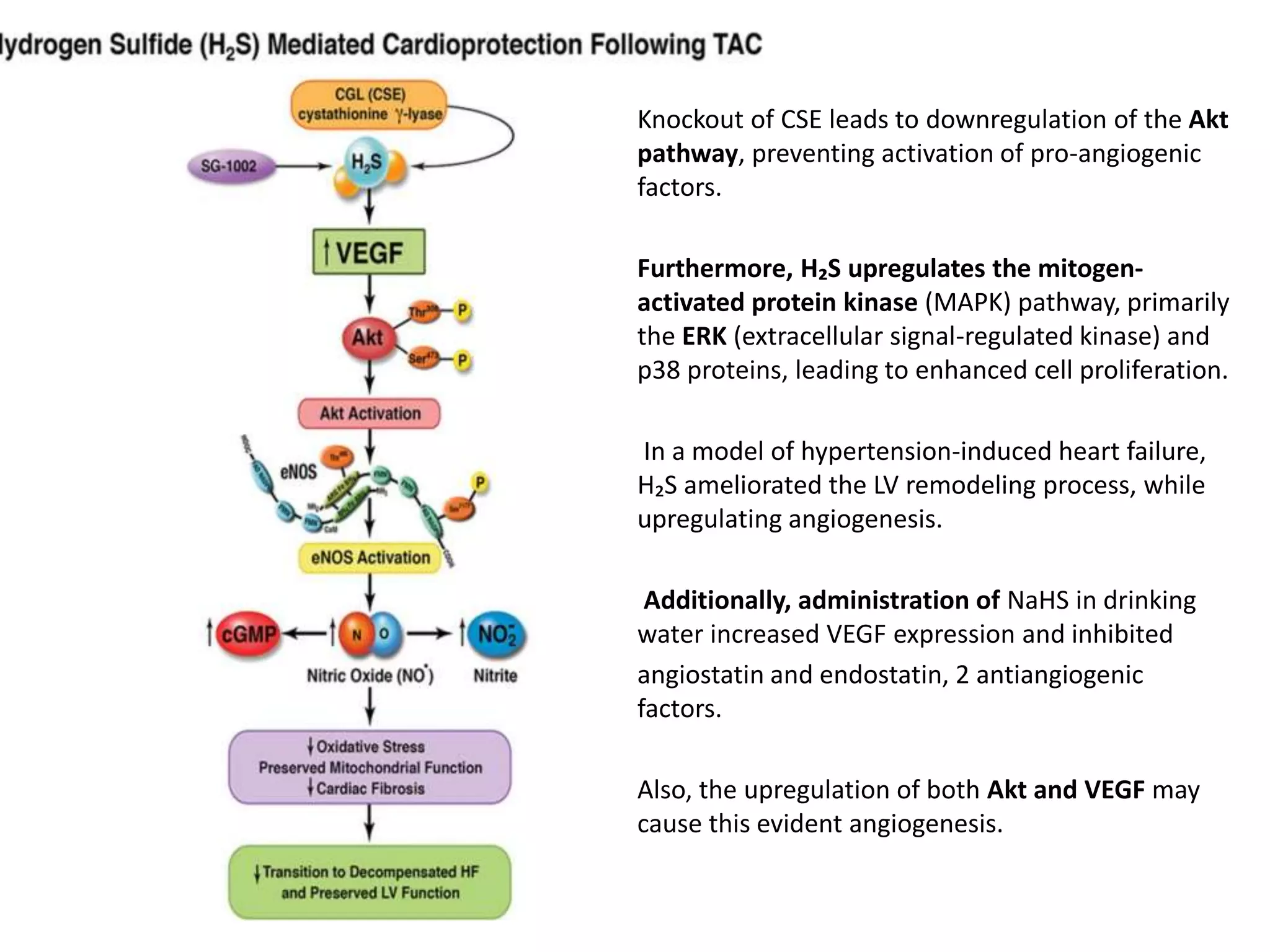
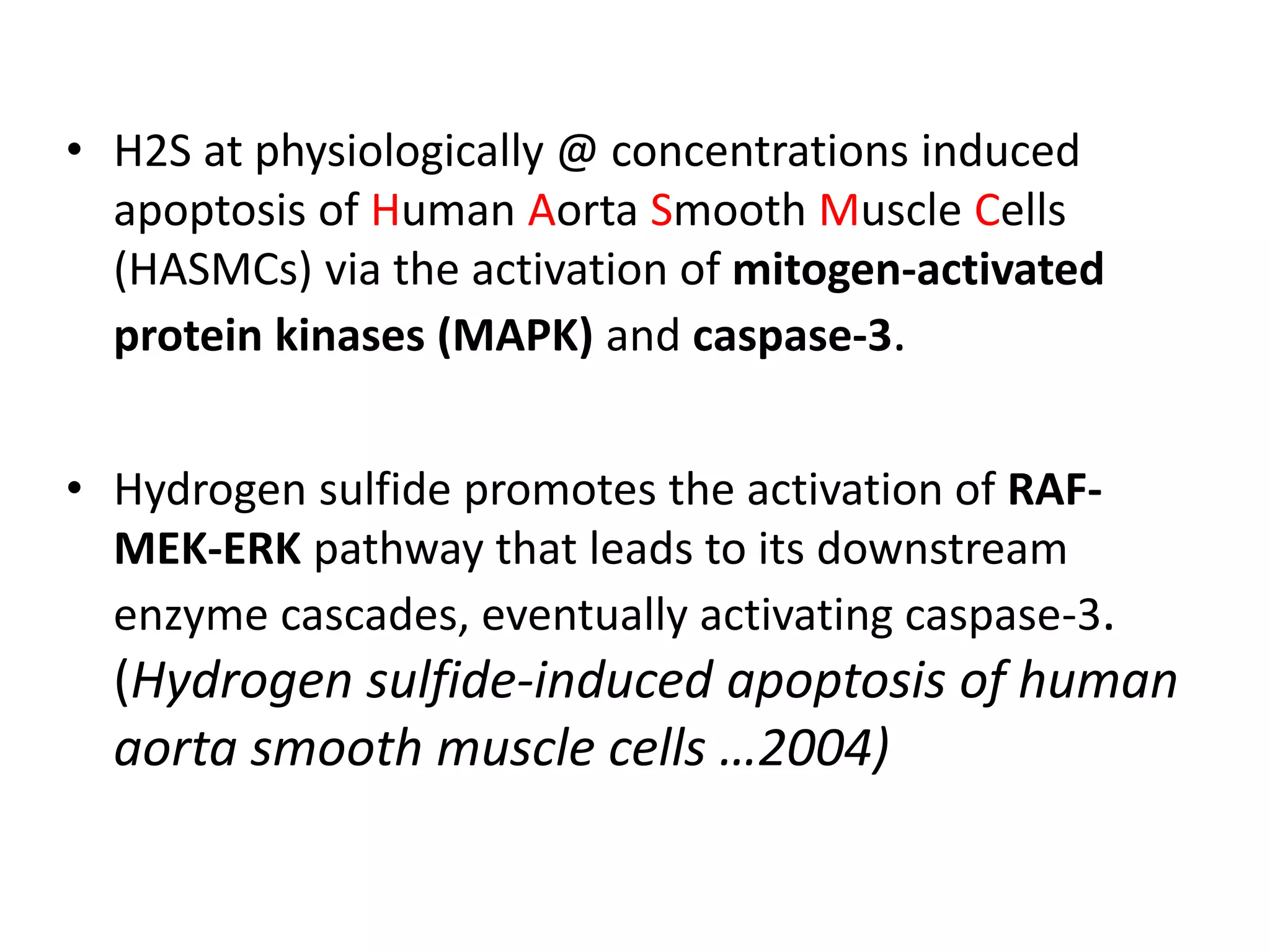
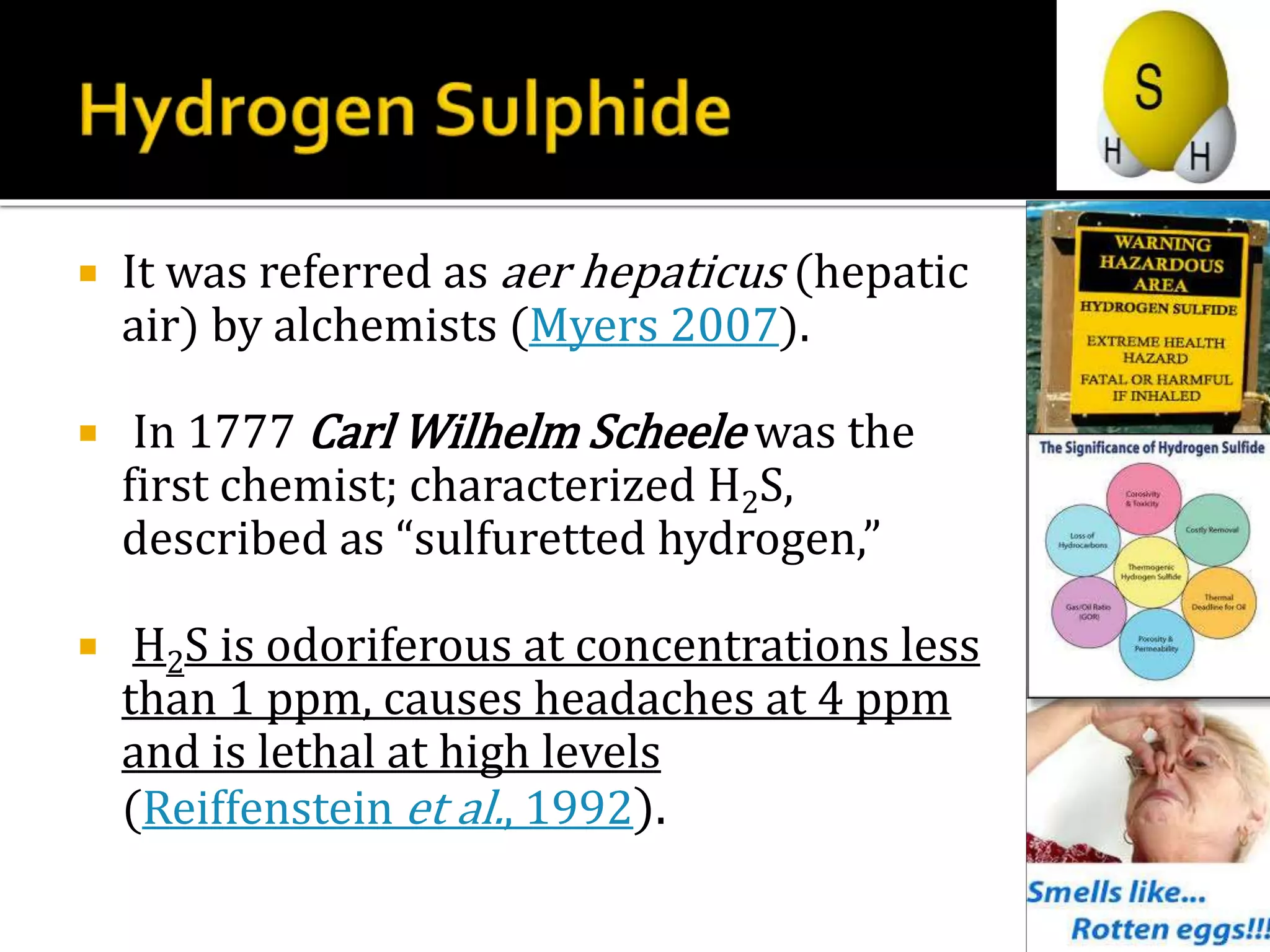
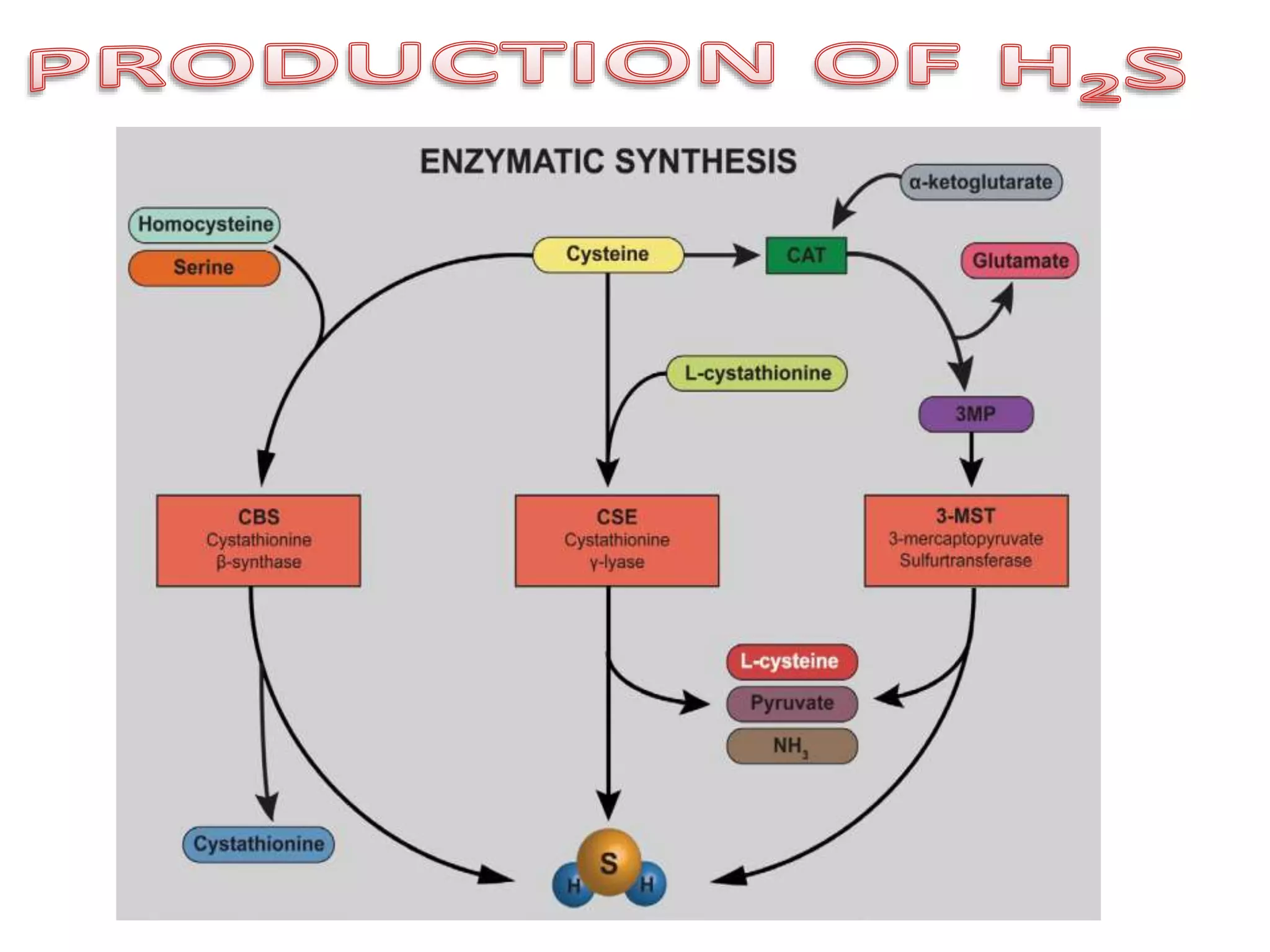
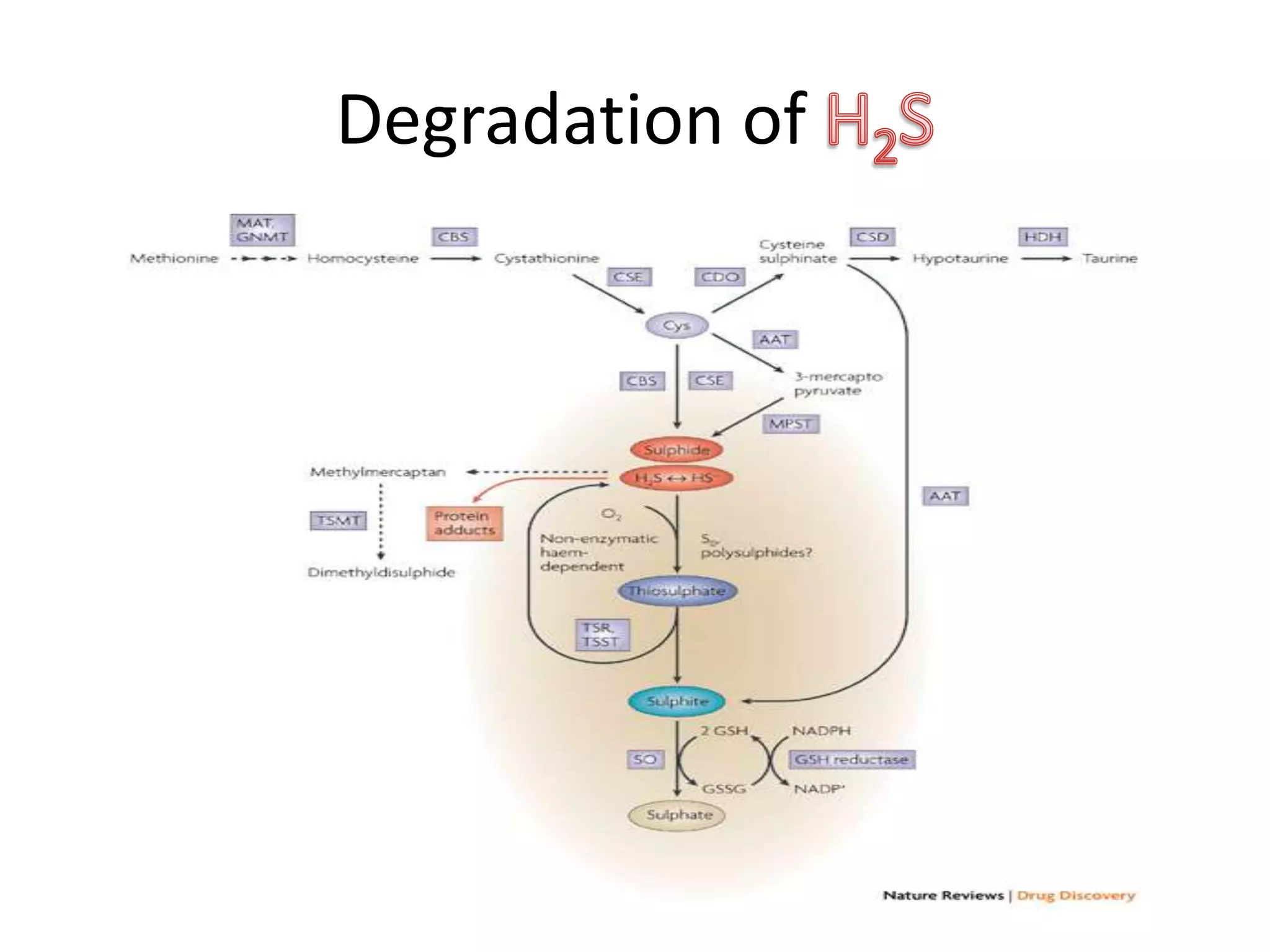
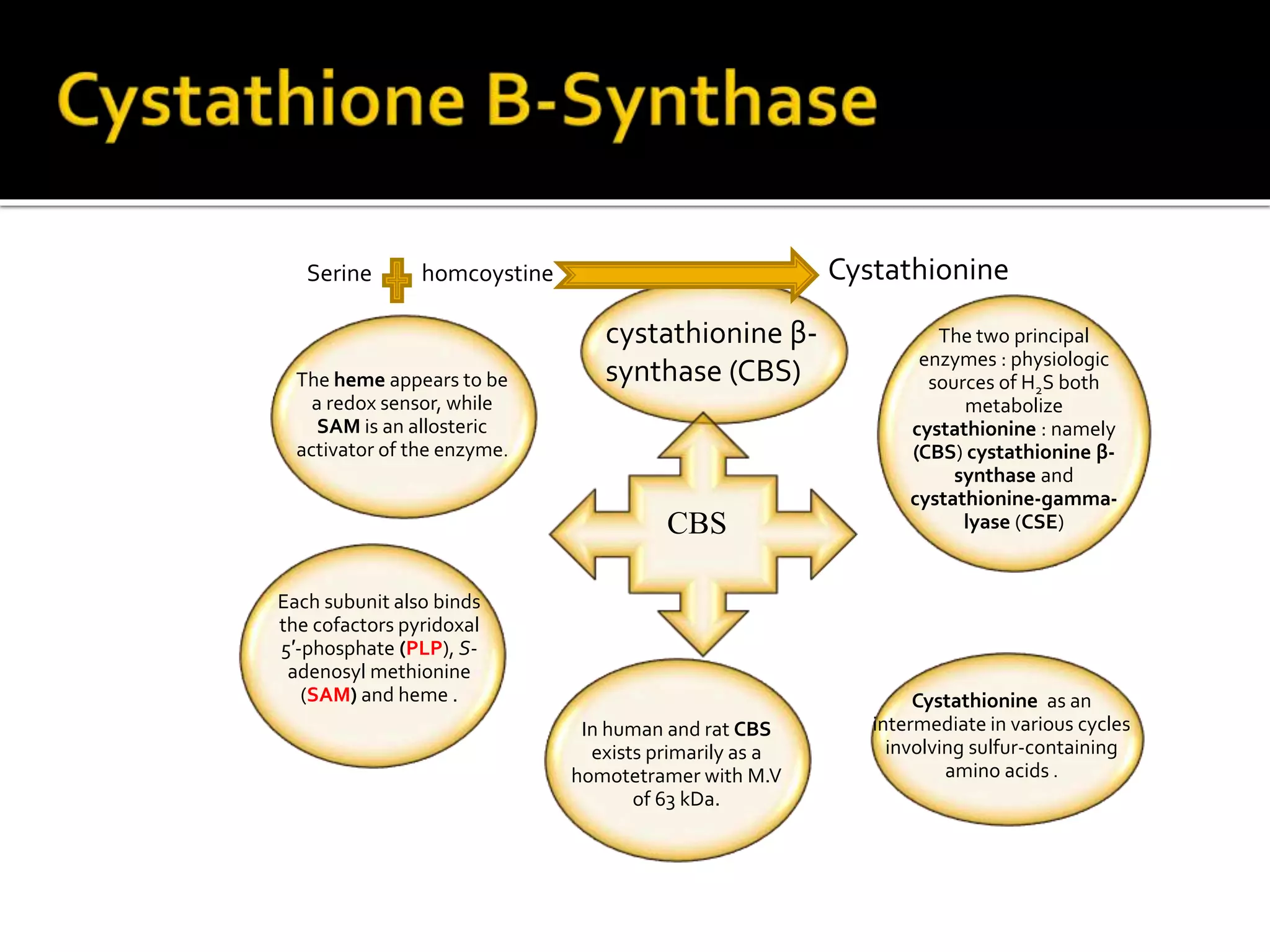
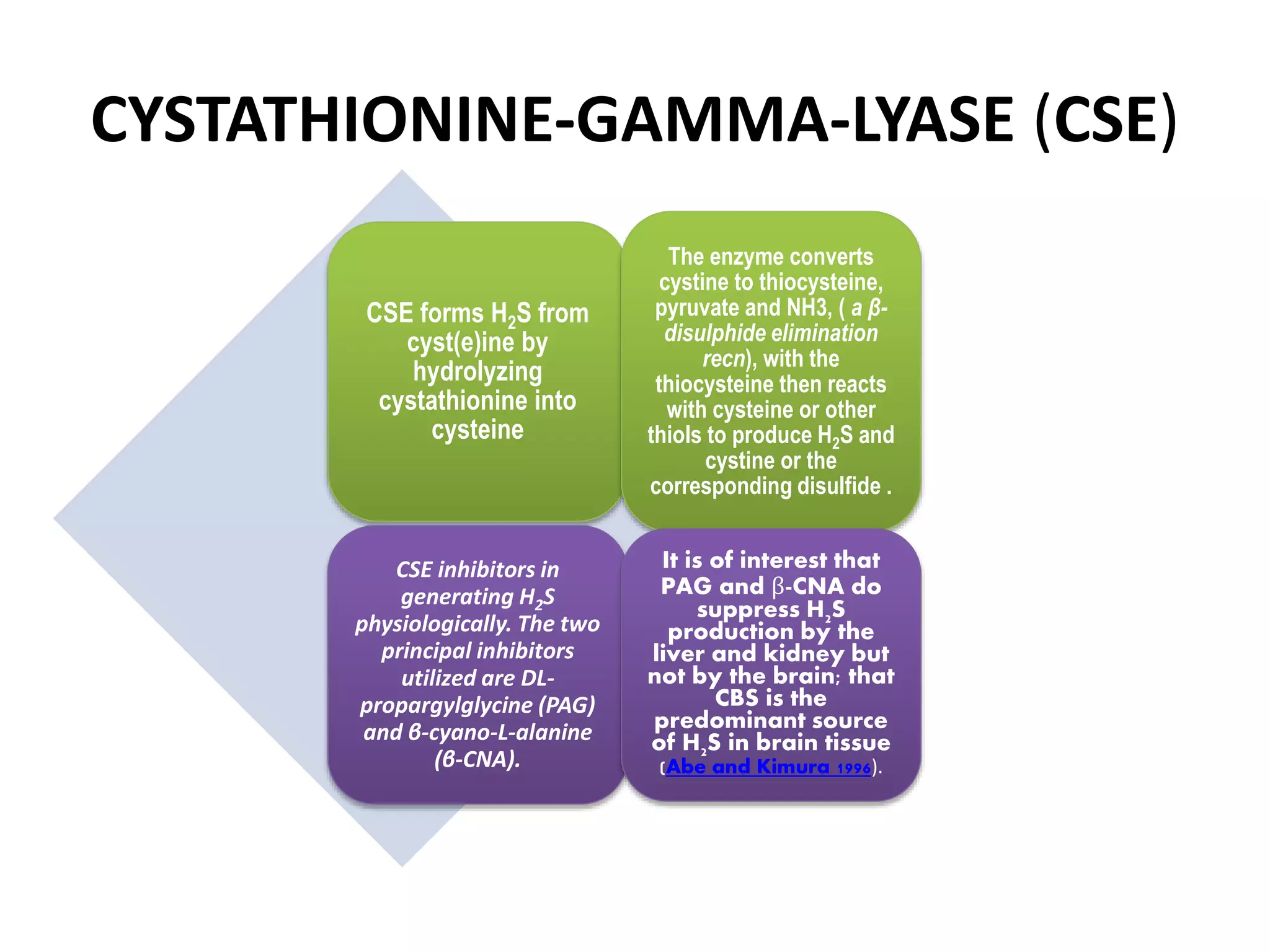
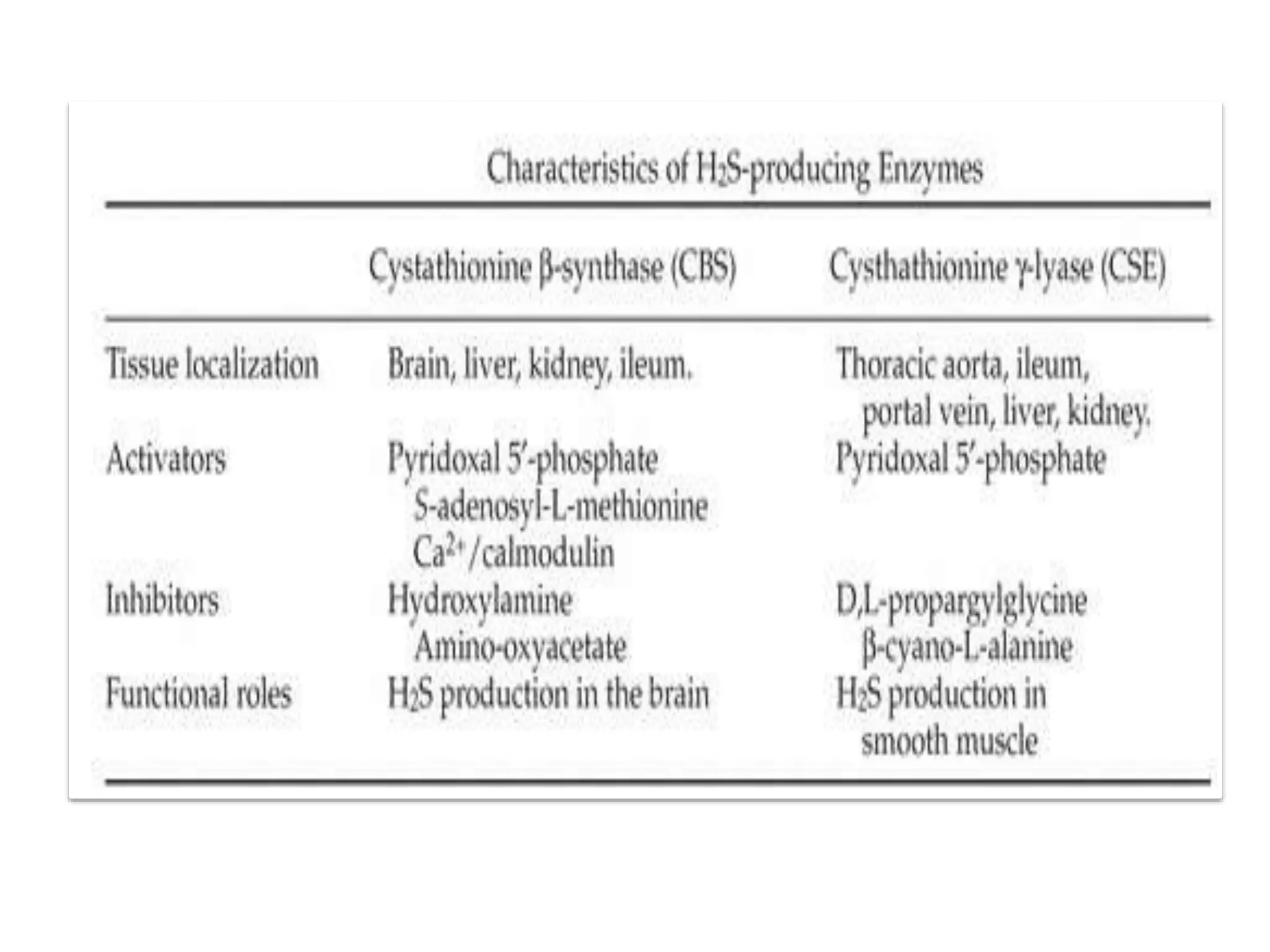
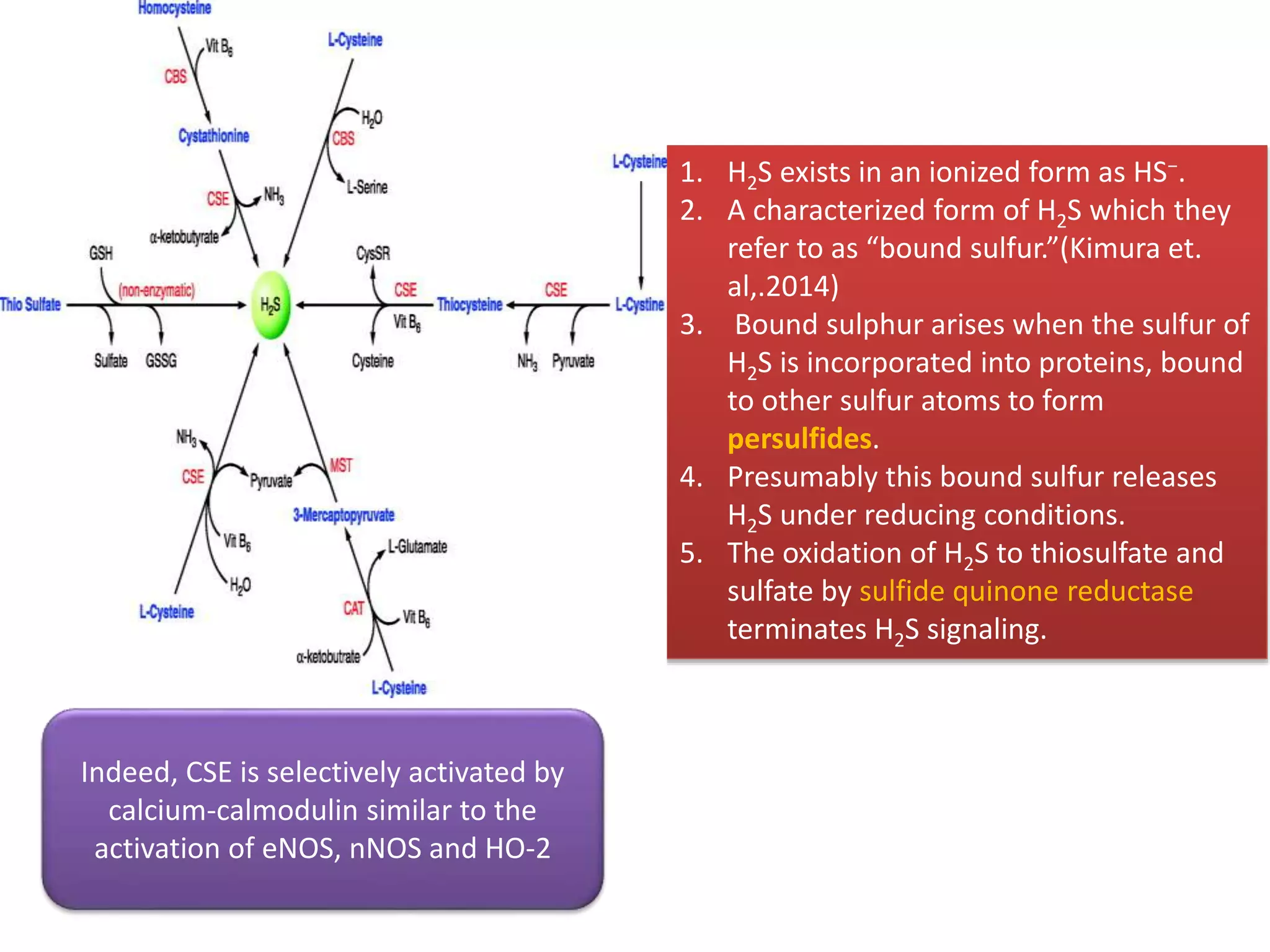
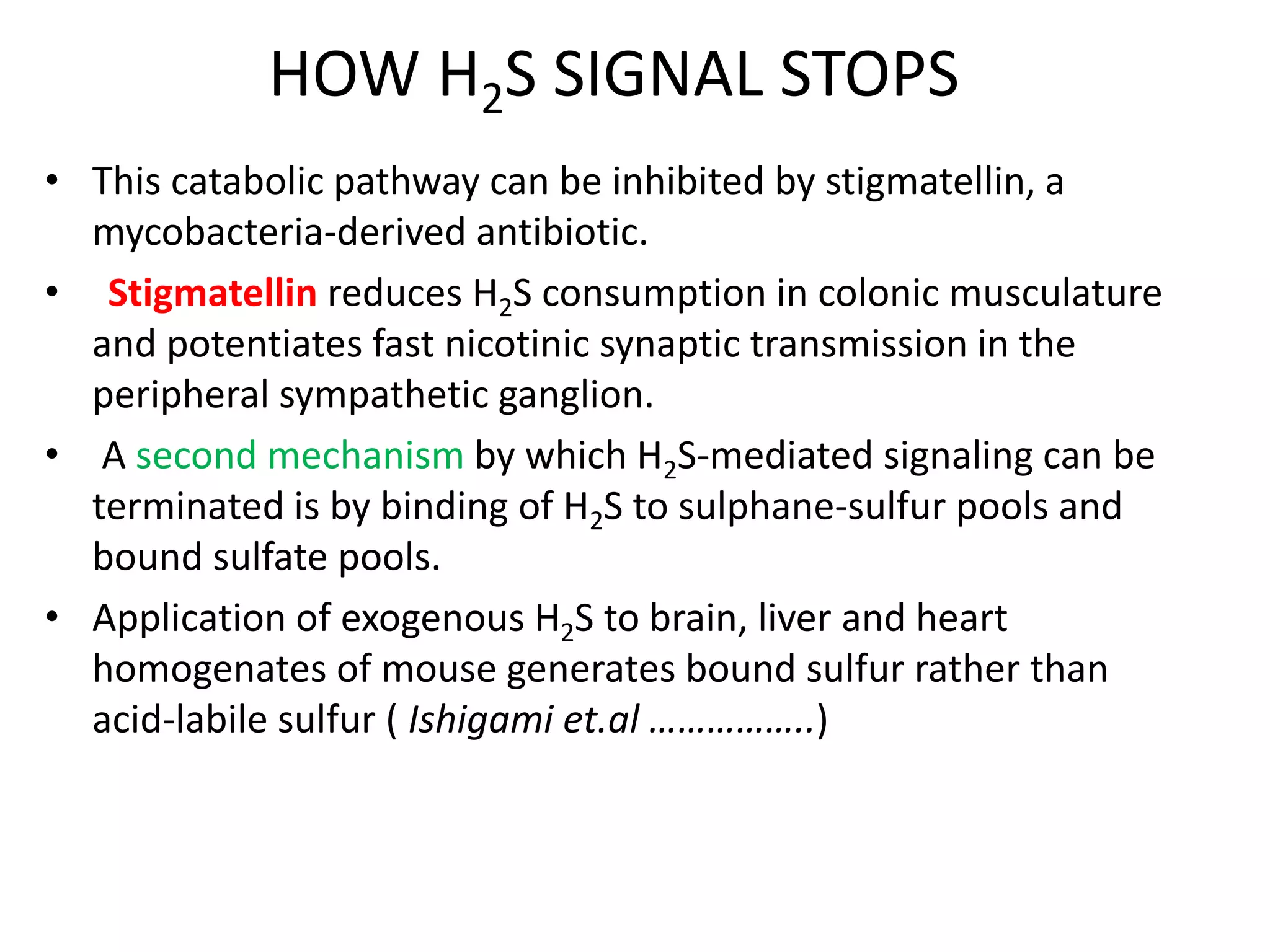
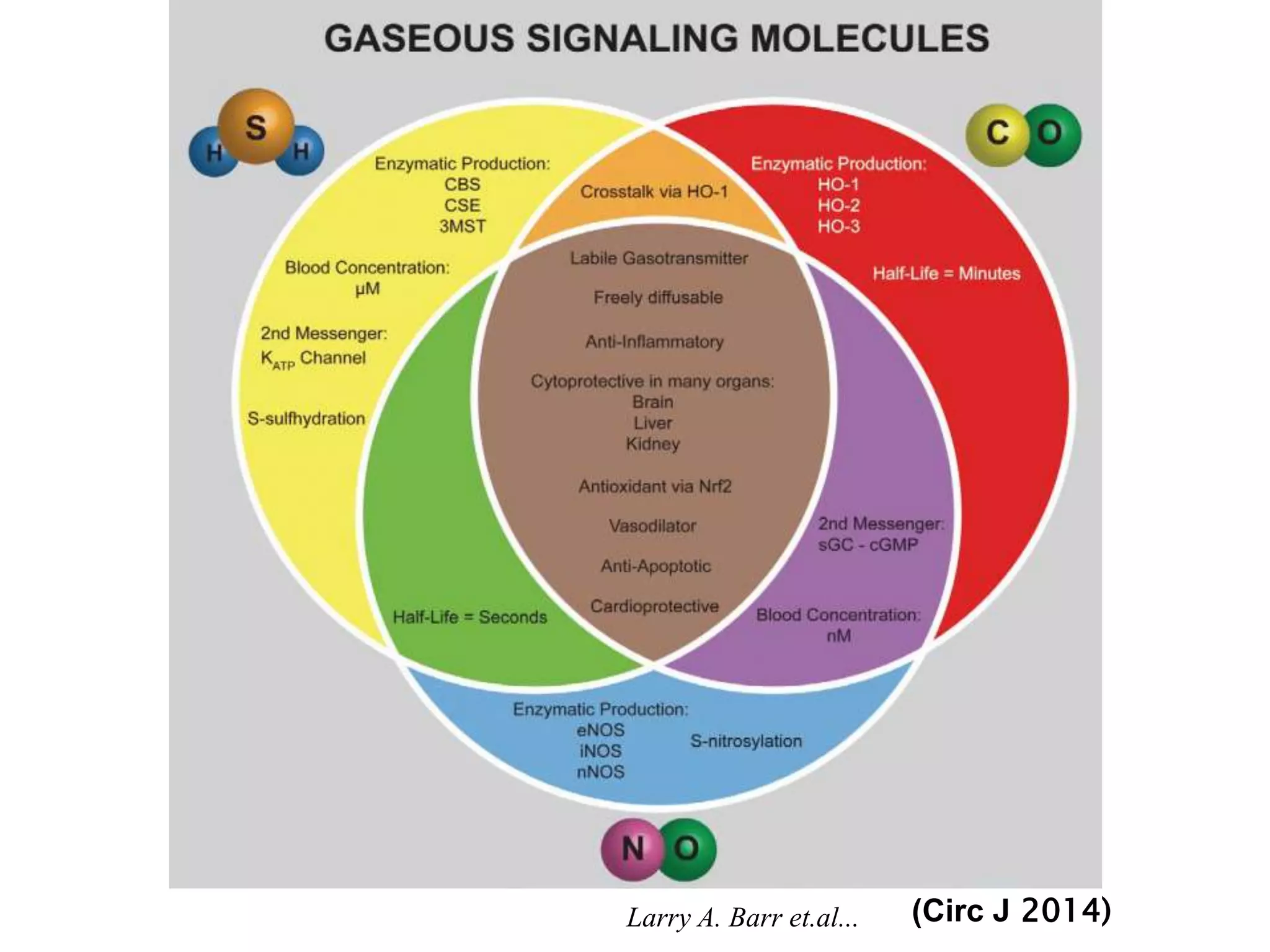
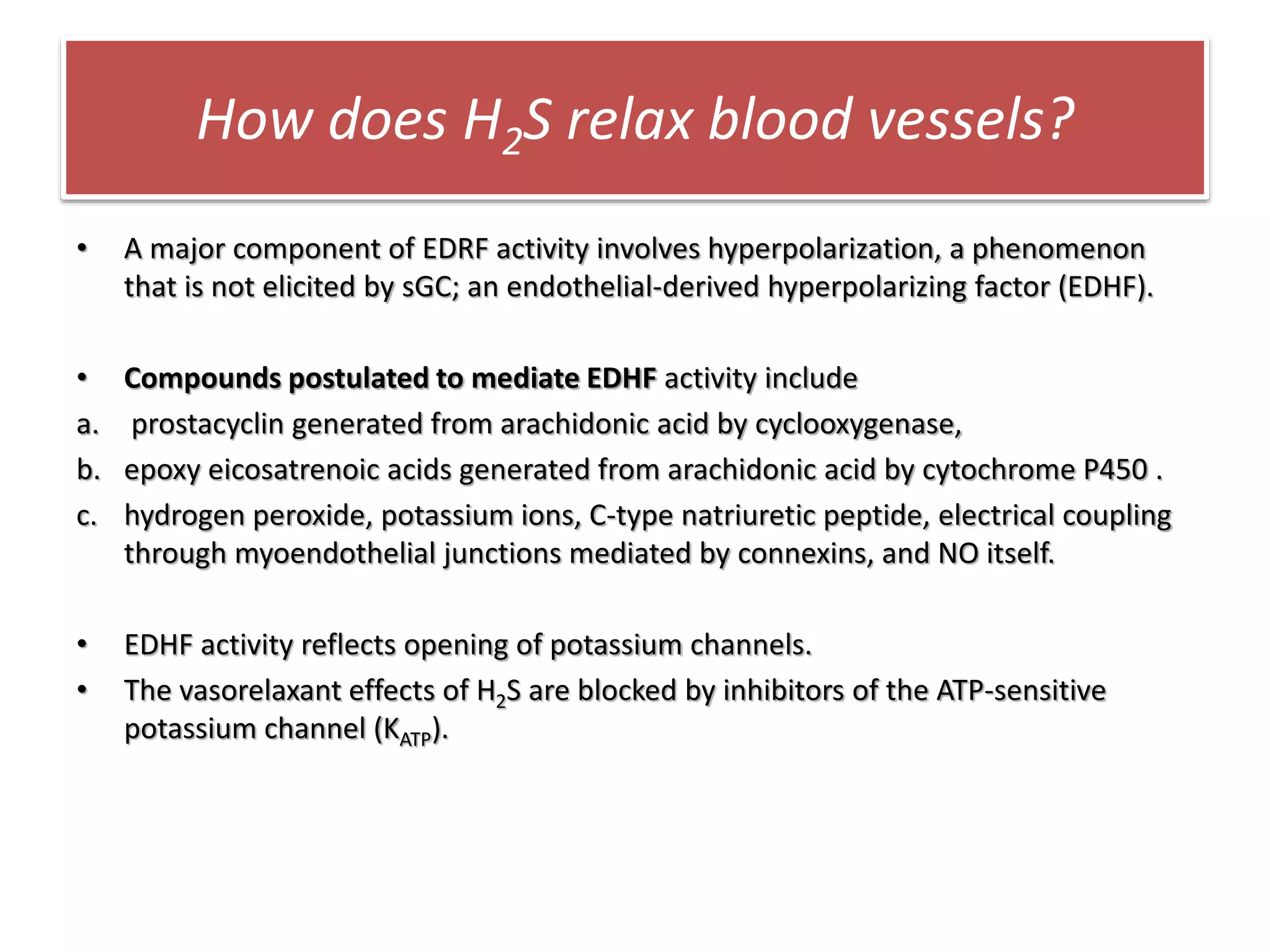
1. Hydrogen sulfide (H2S) exists in an ionized form as HS- and as "bound sulfur" incorporated into proteins.
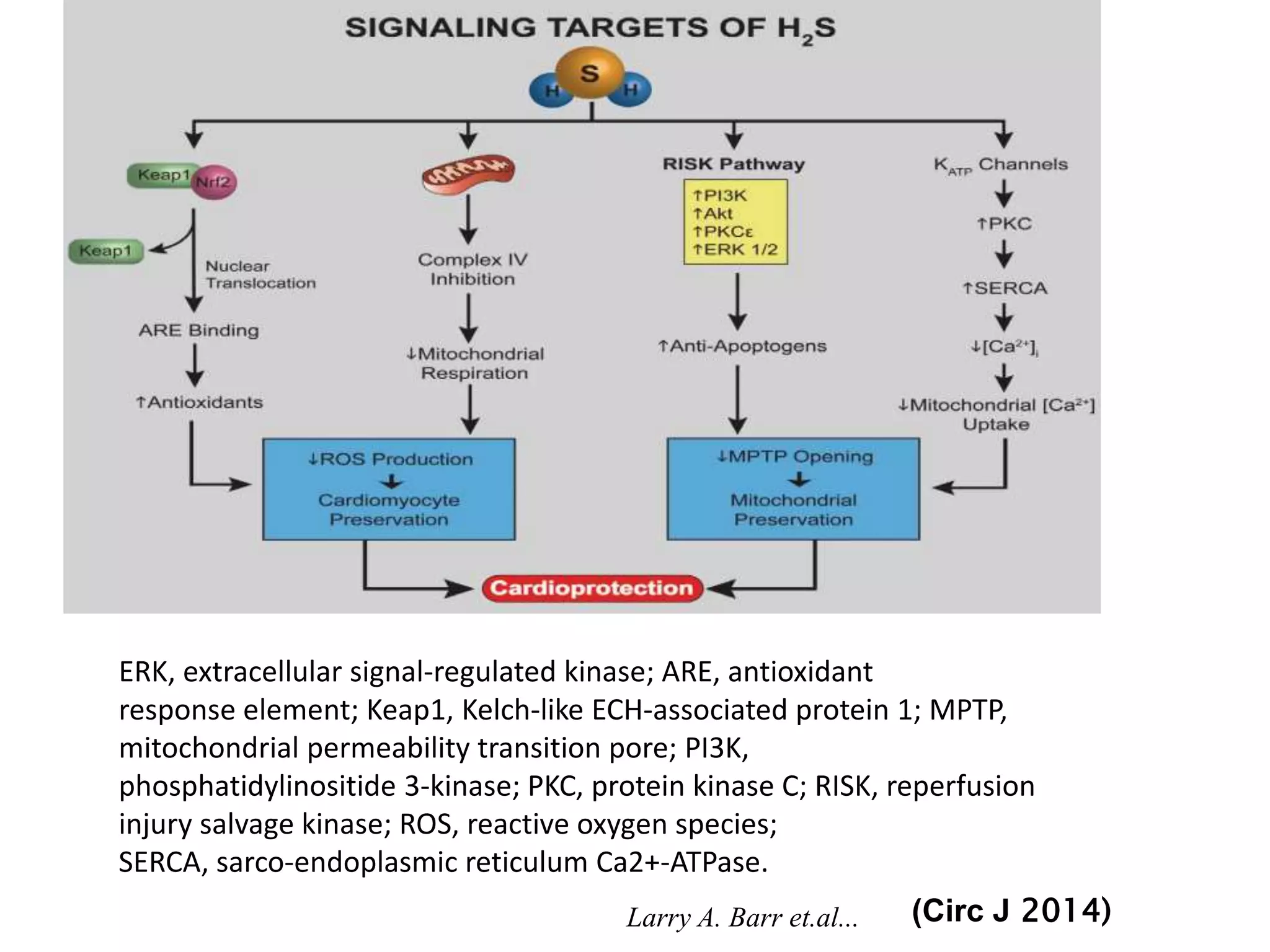
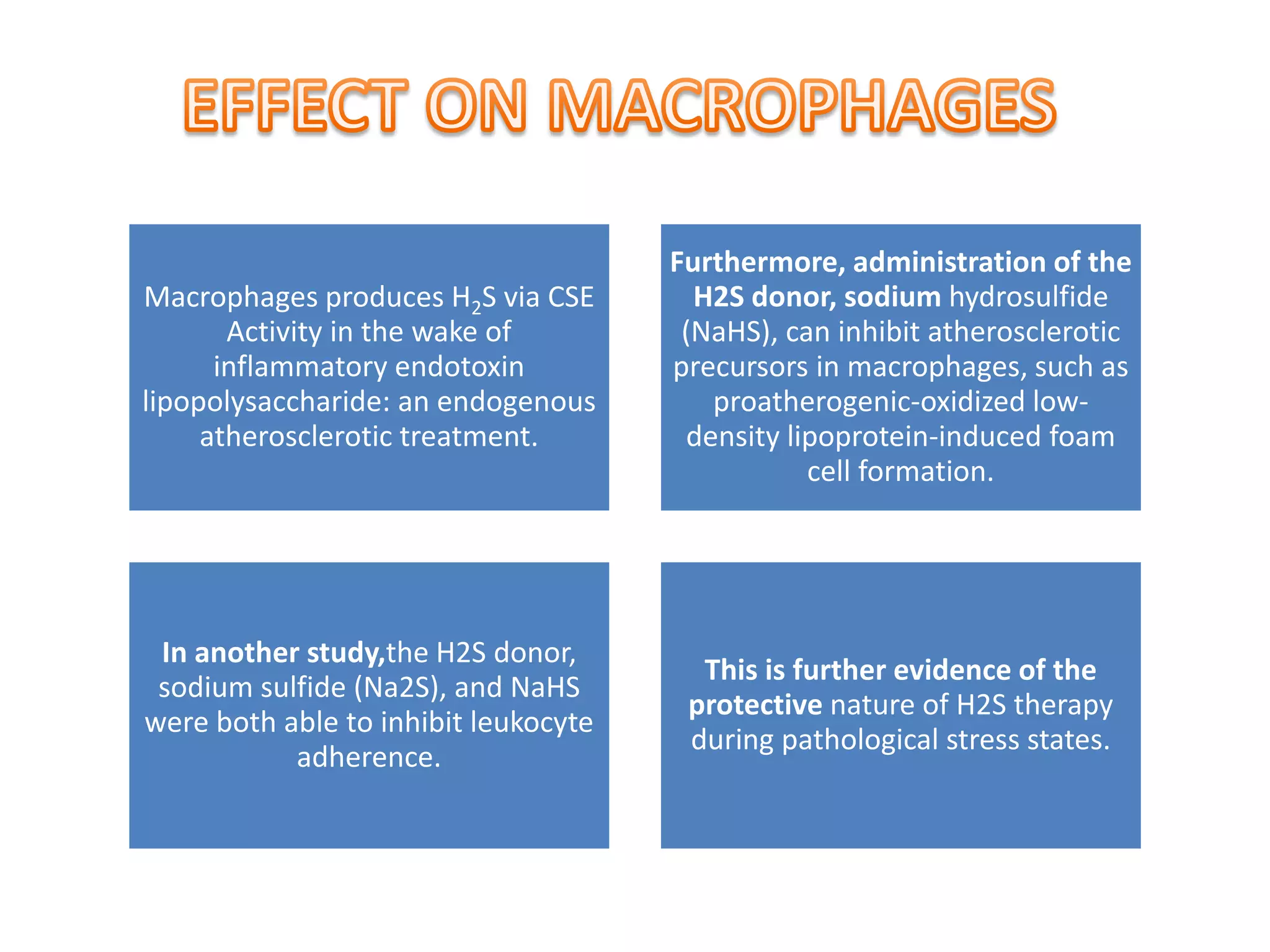
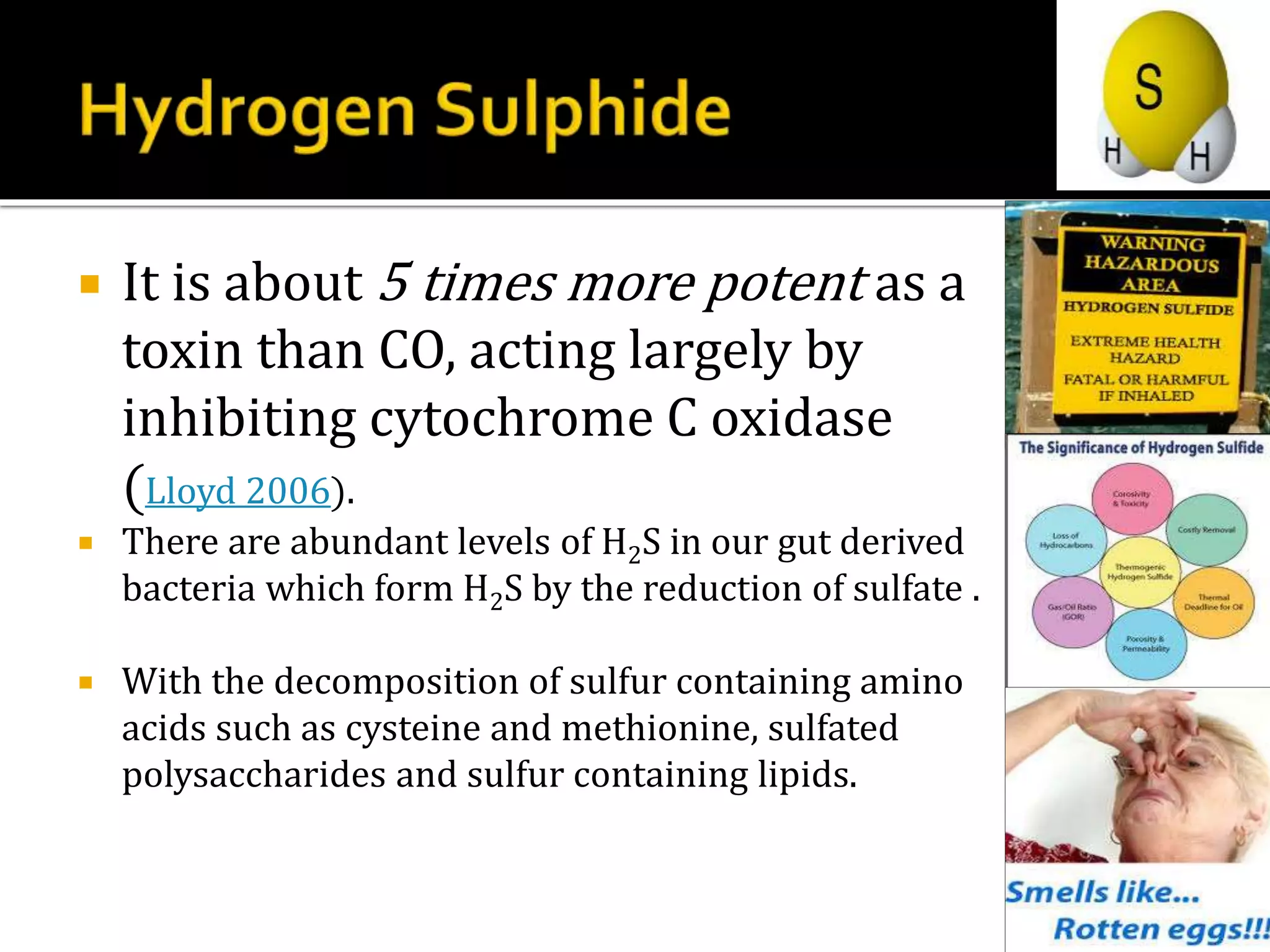
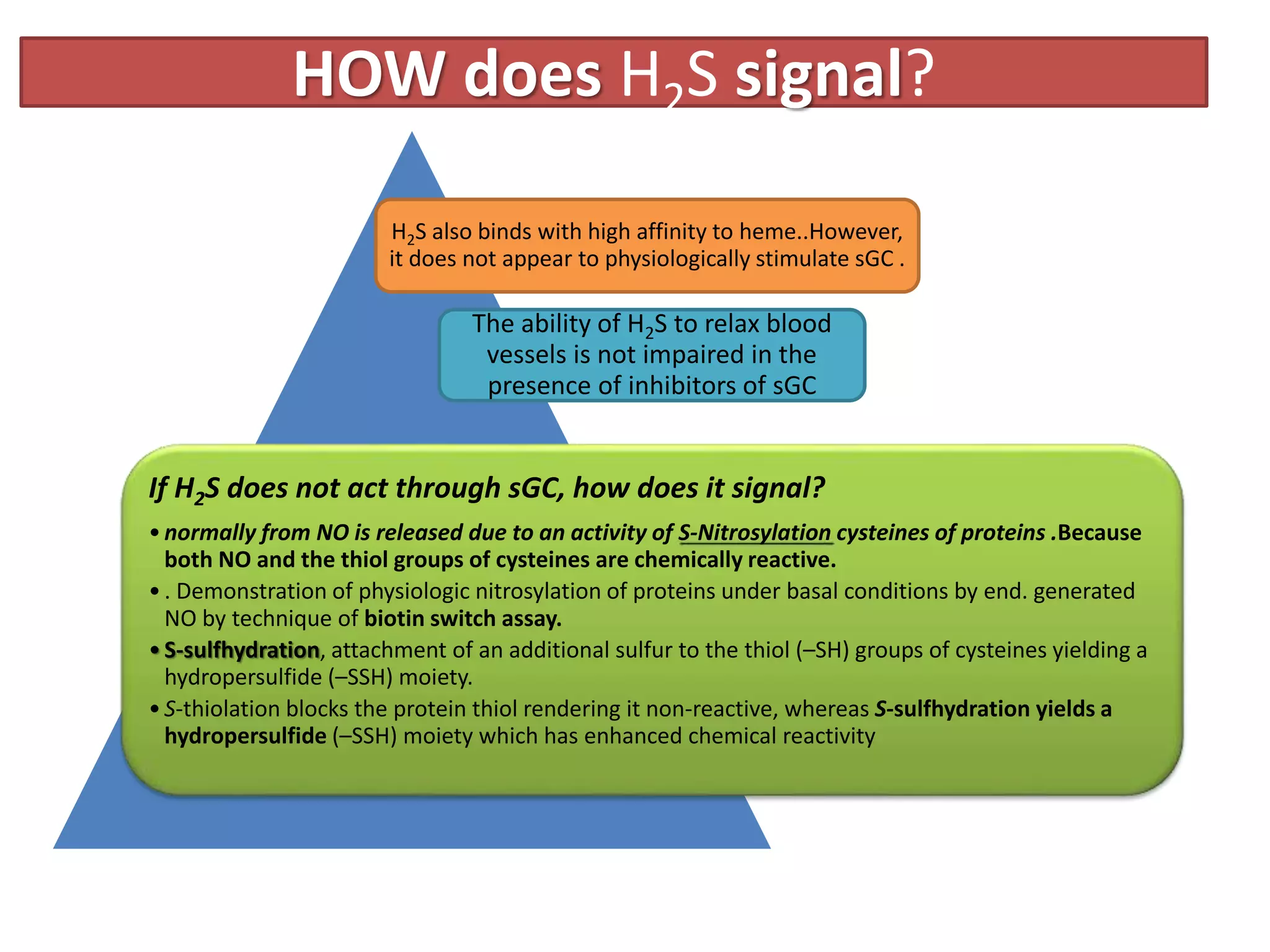
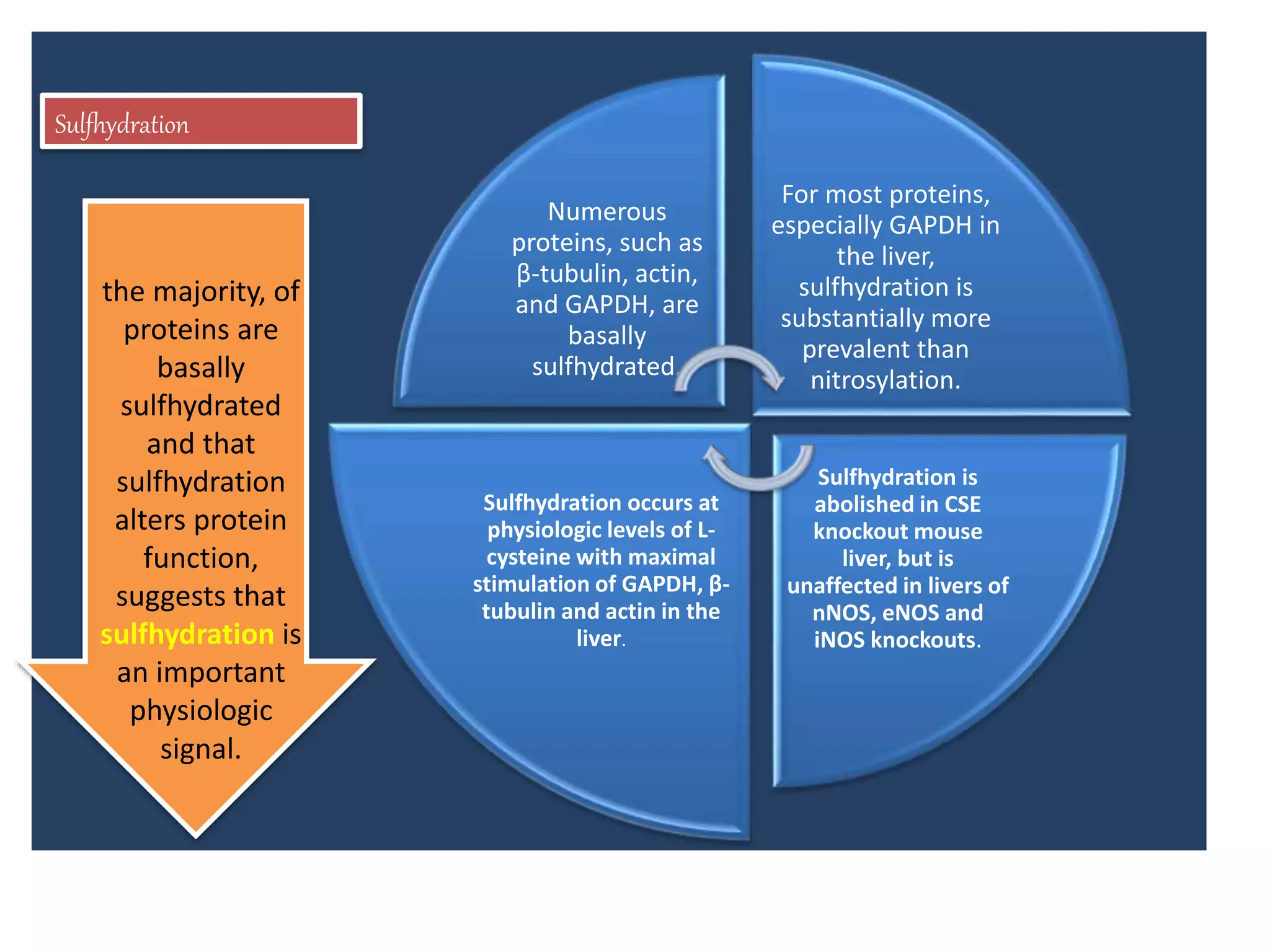
2. H2S signaling occurs through protein sulfhydration, the addition of sulfur to cysteine residues, altering protein function. This is more prevalent than nitric oxide protein nitrosylation.
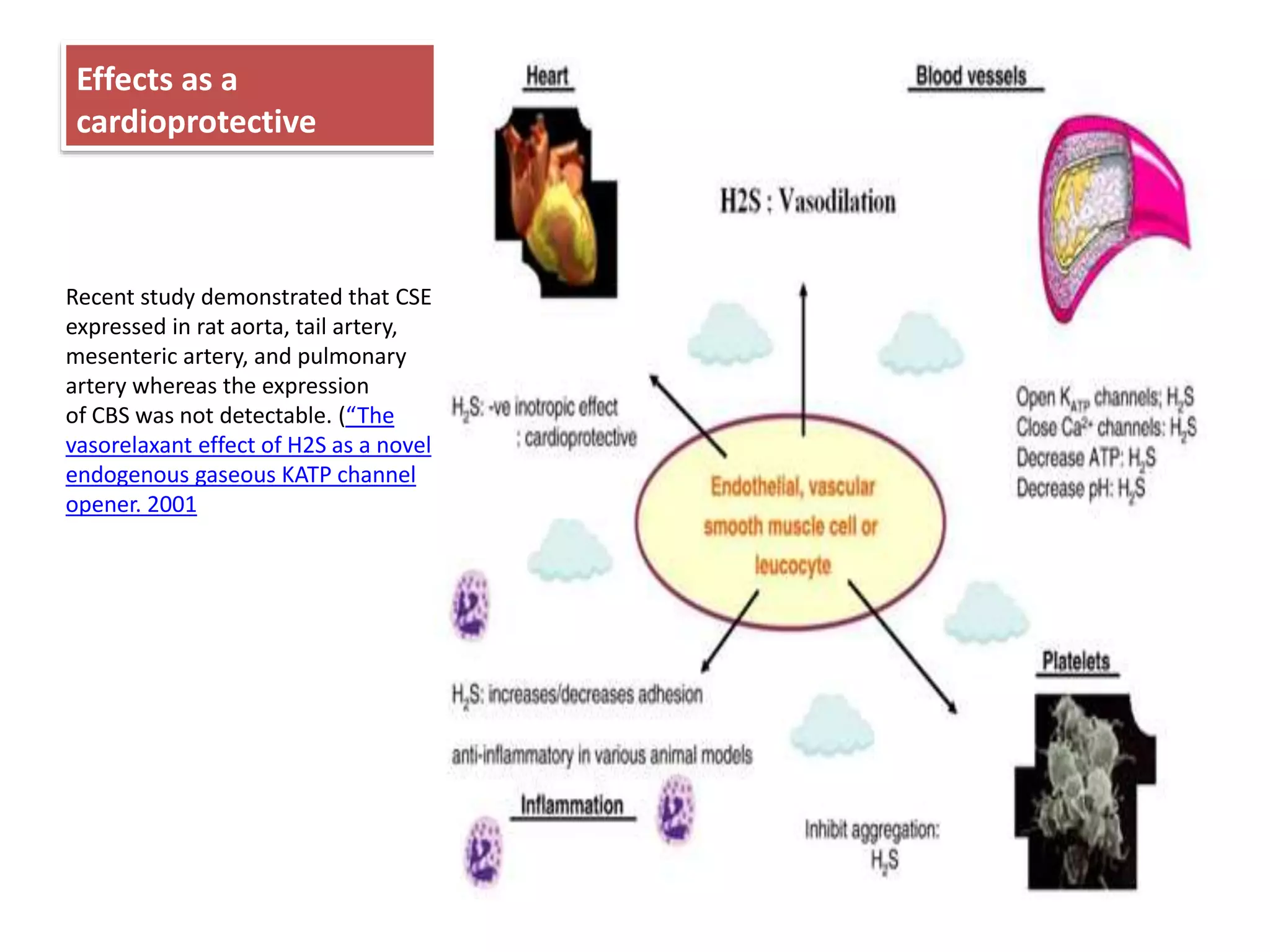
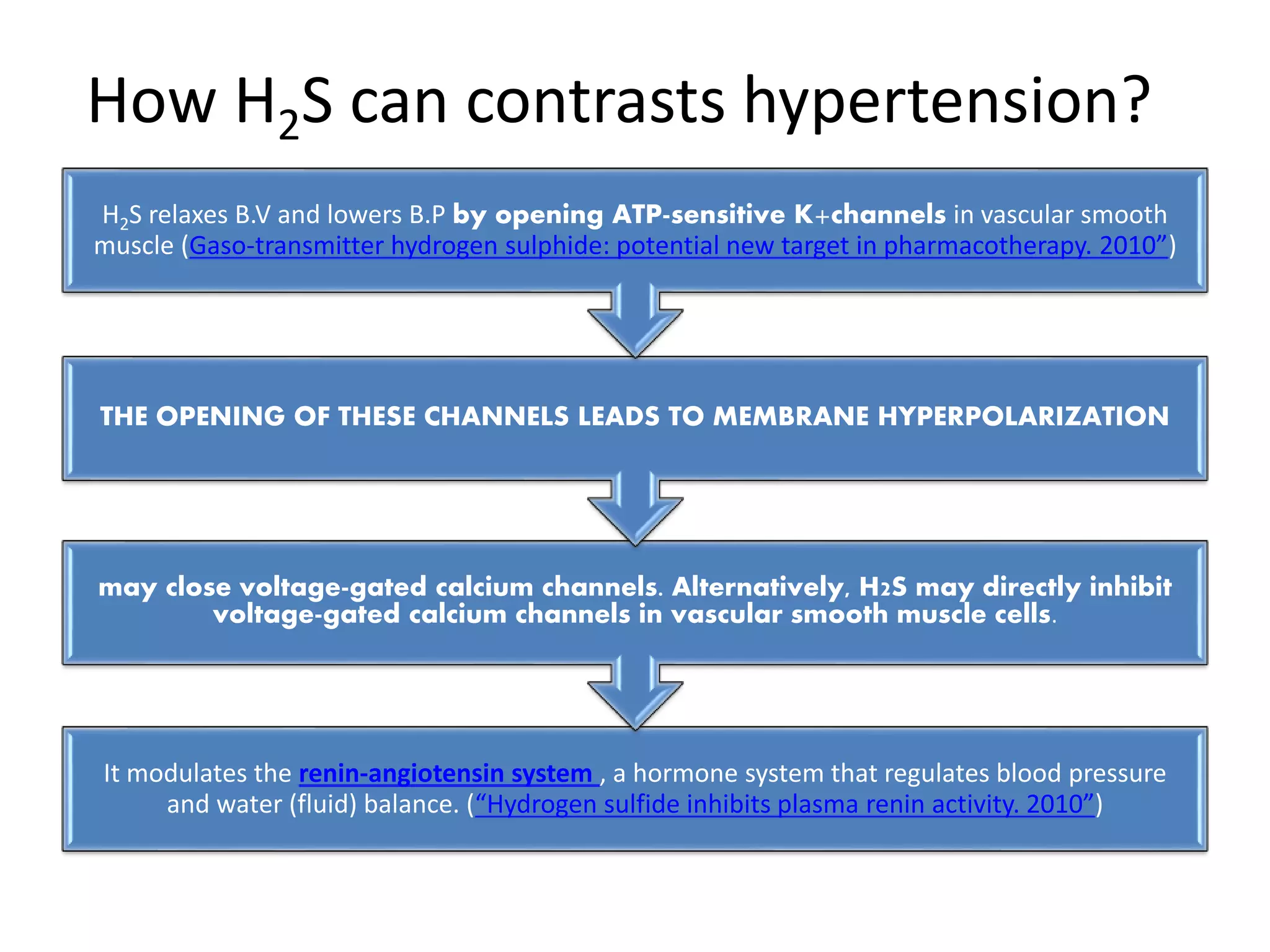
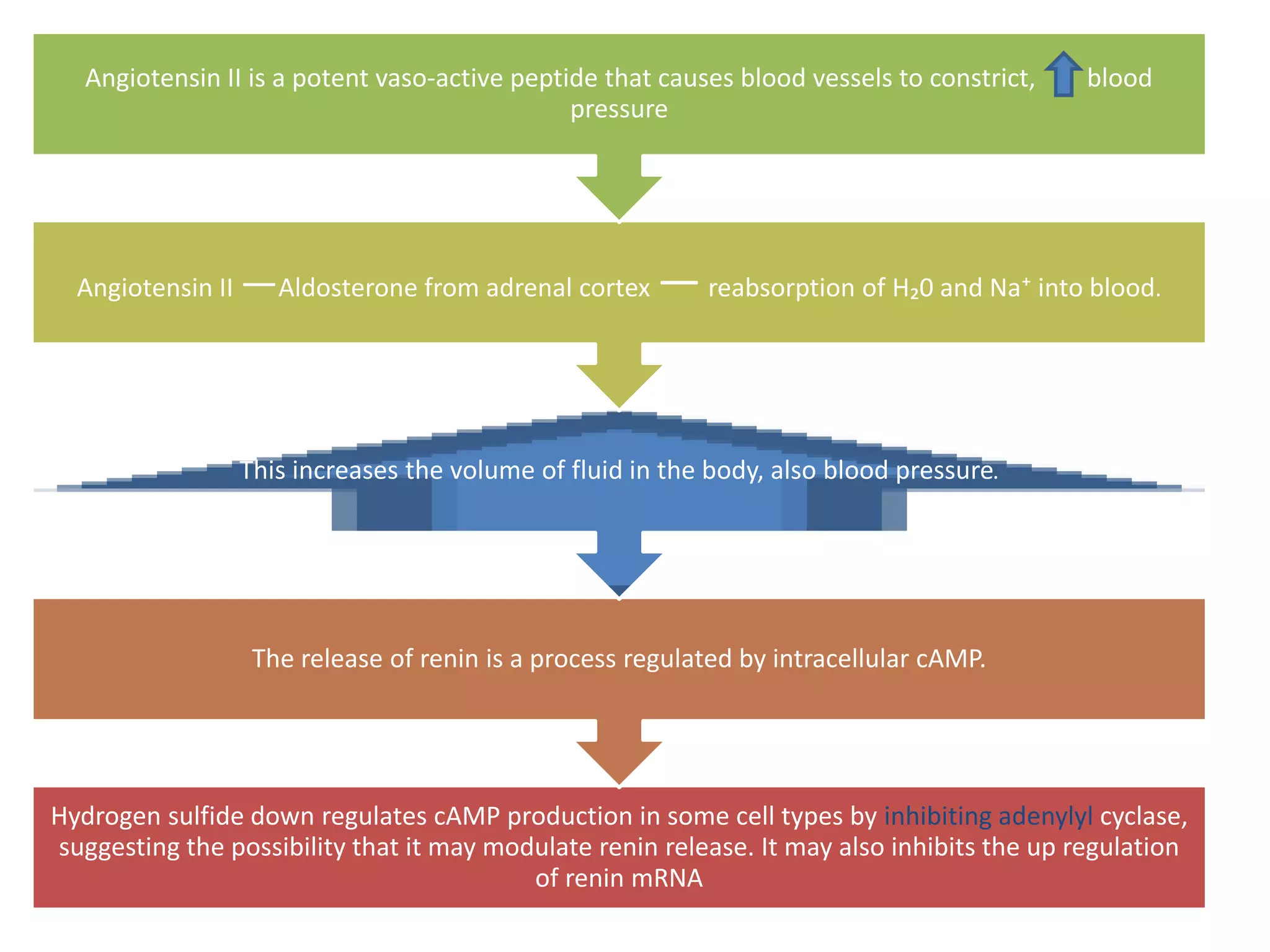
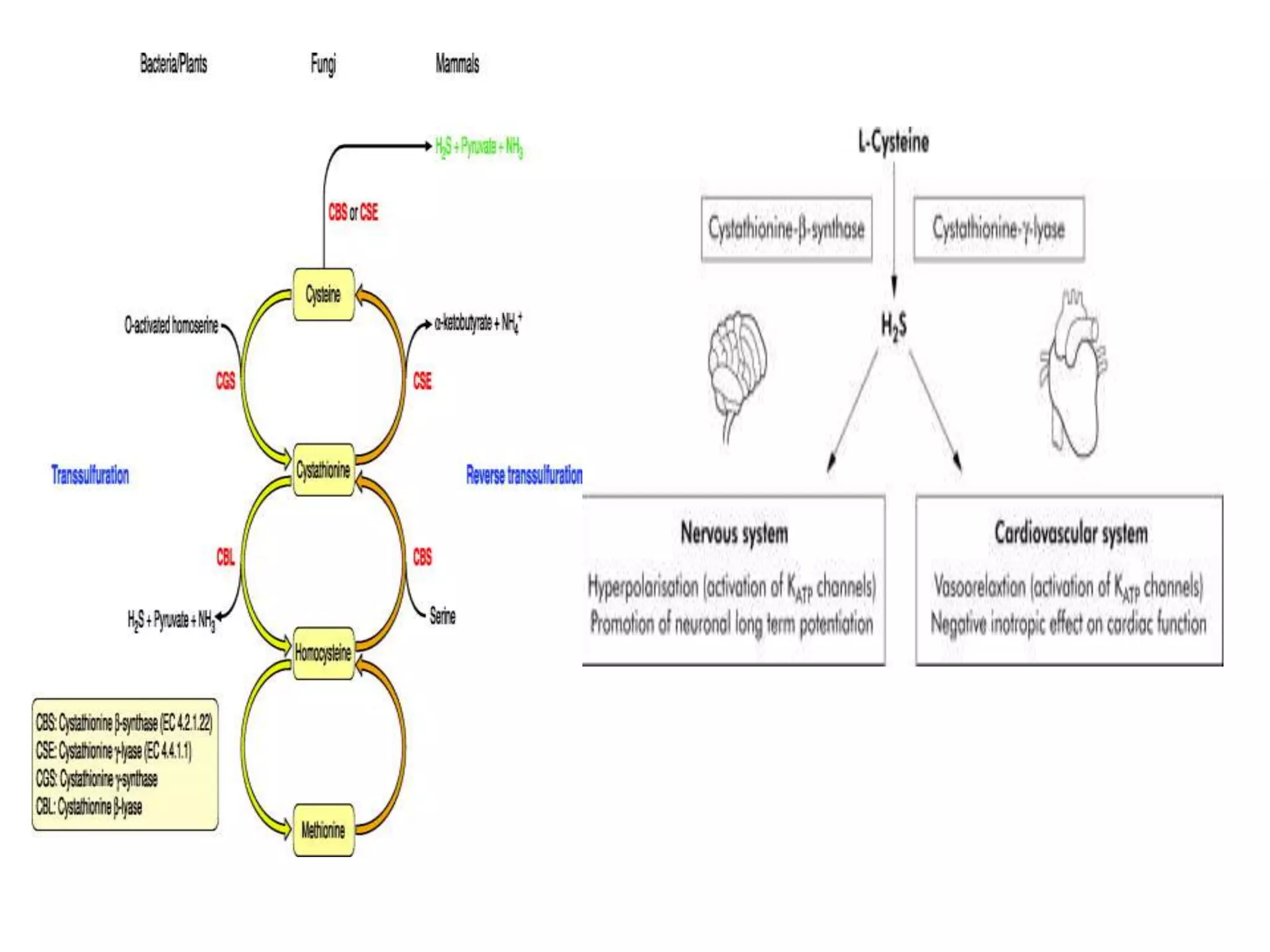
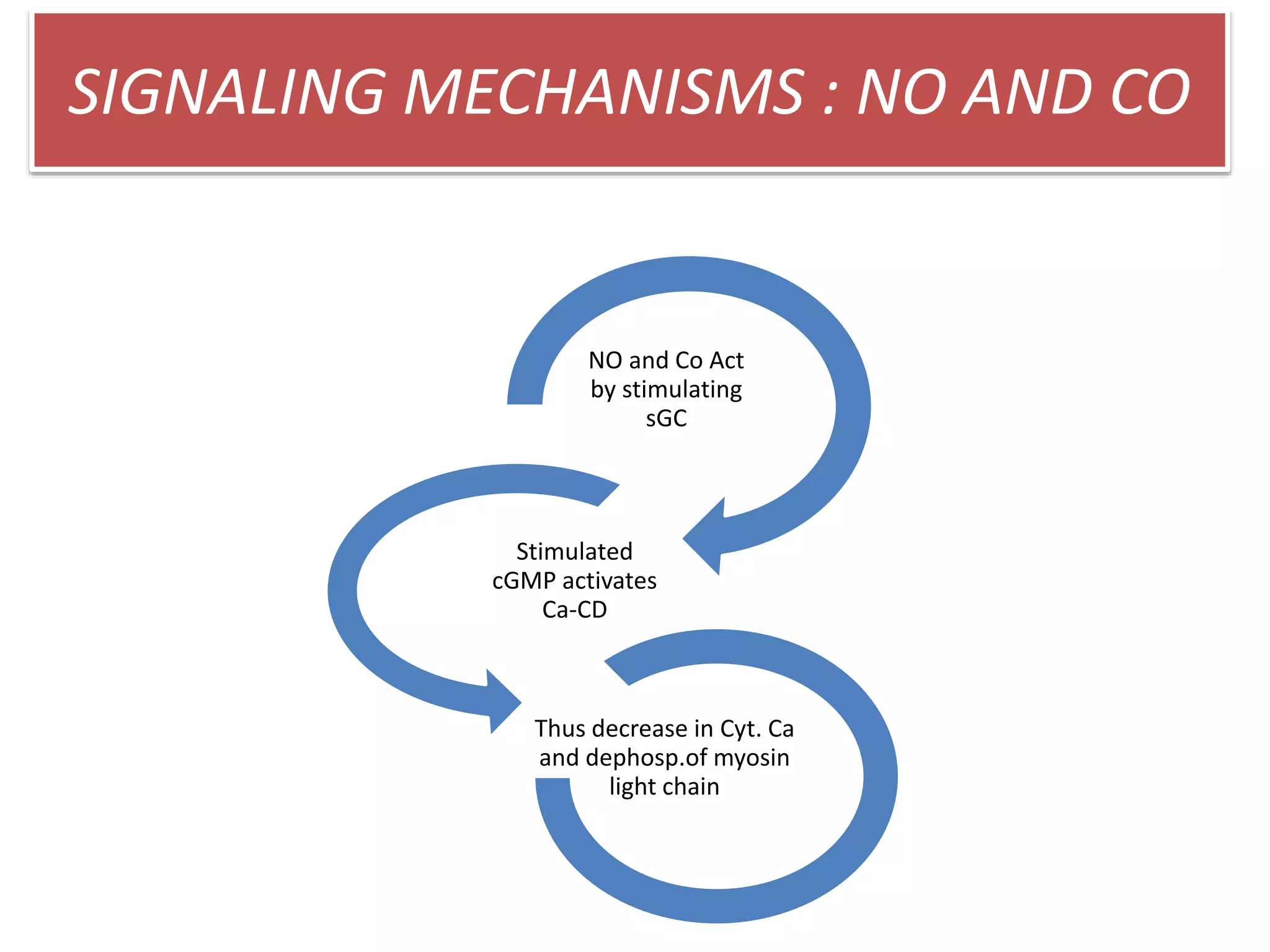
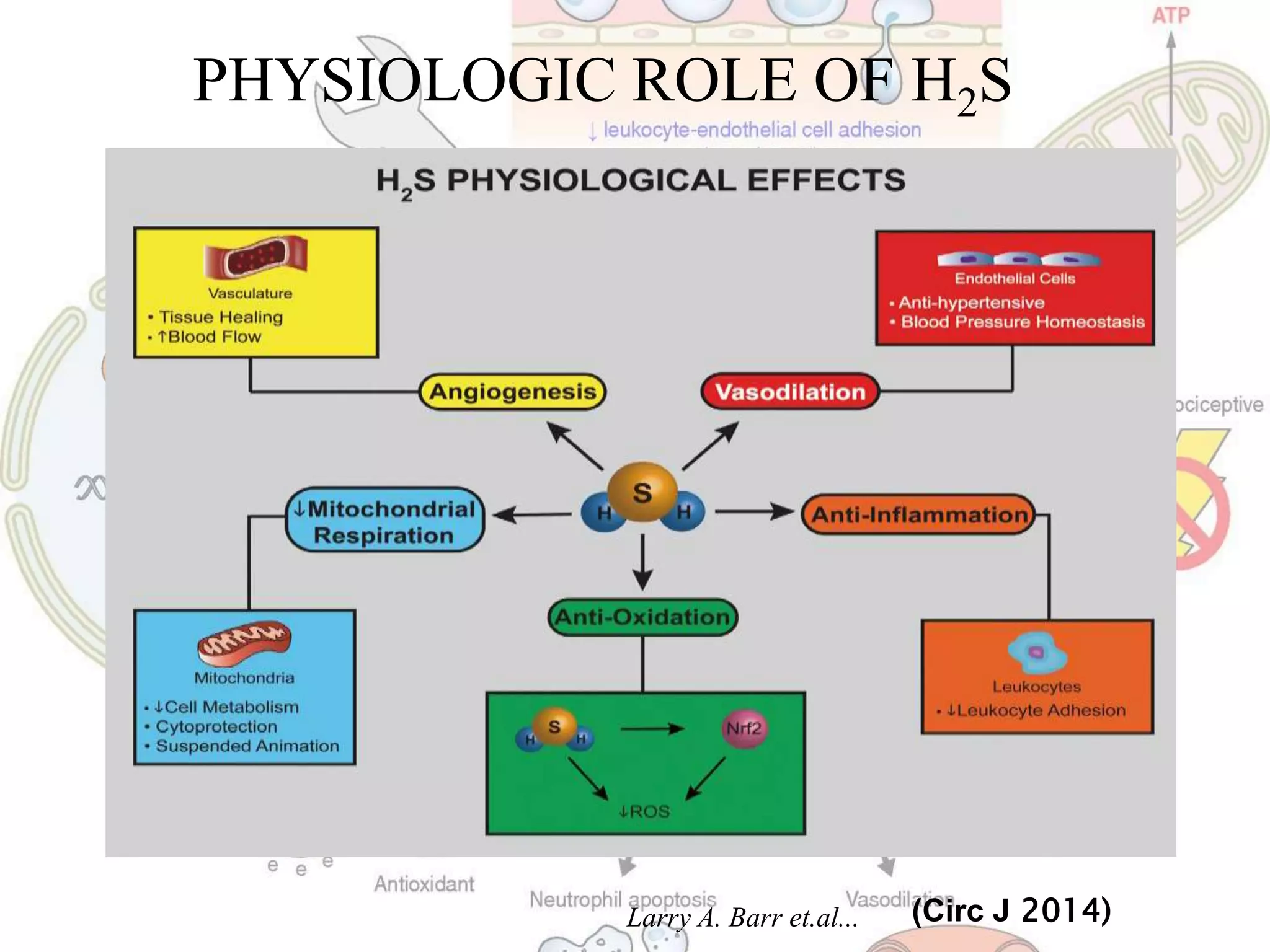
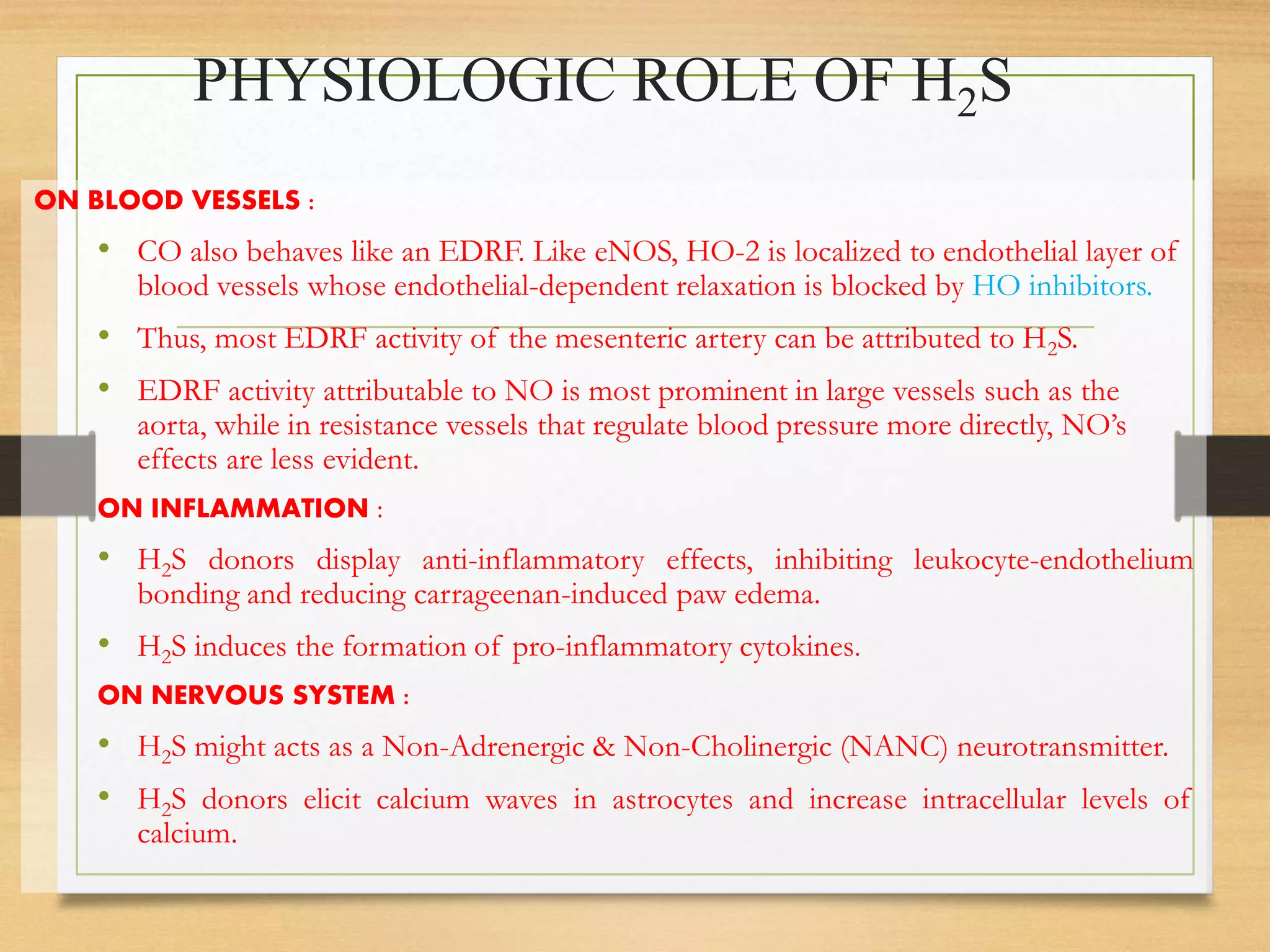
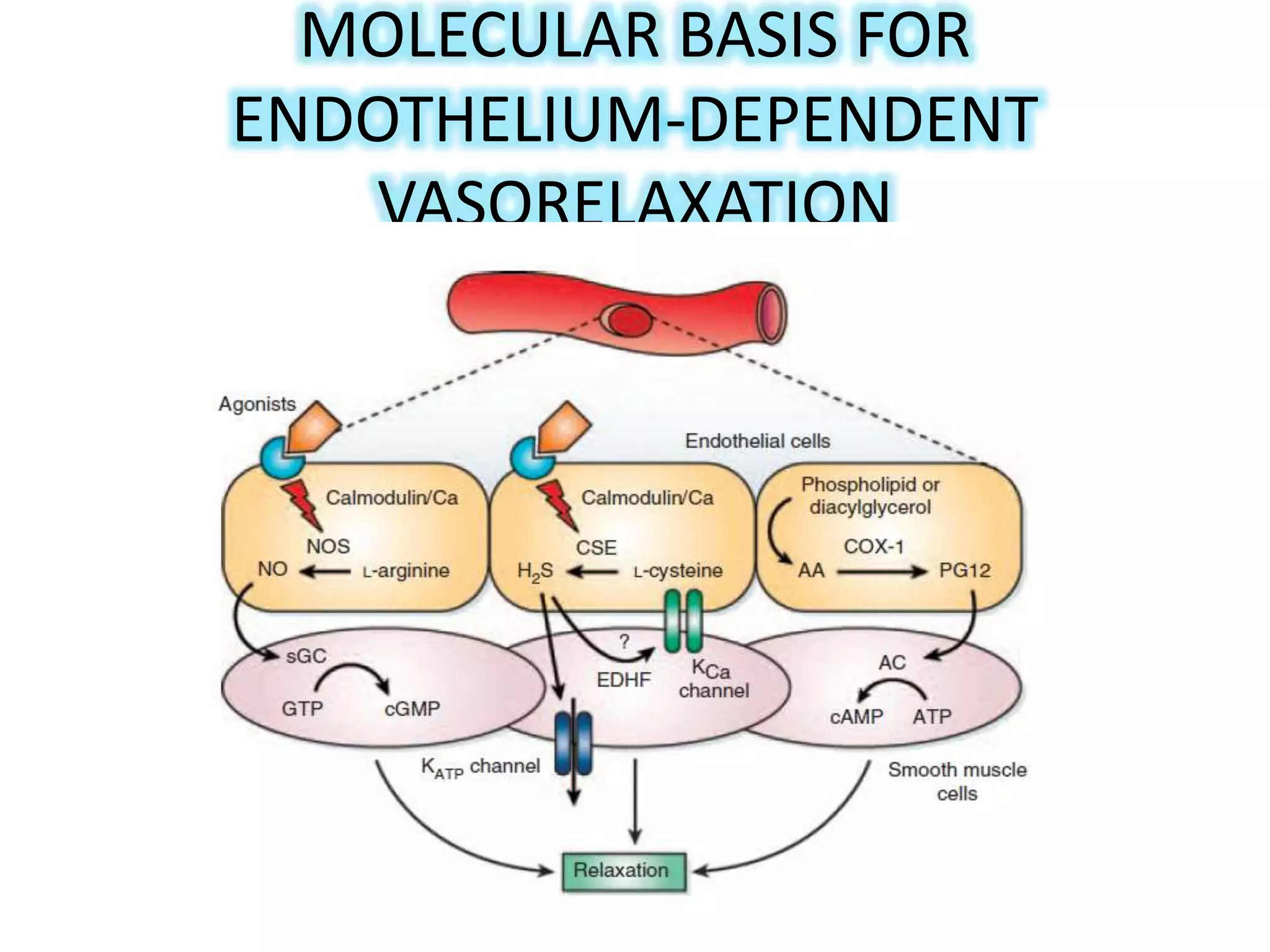
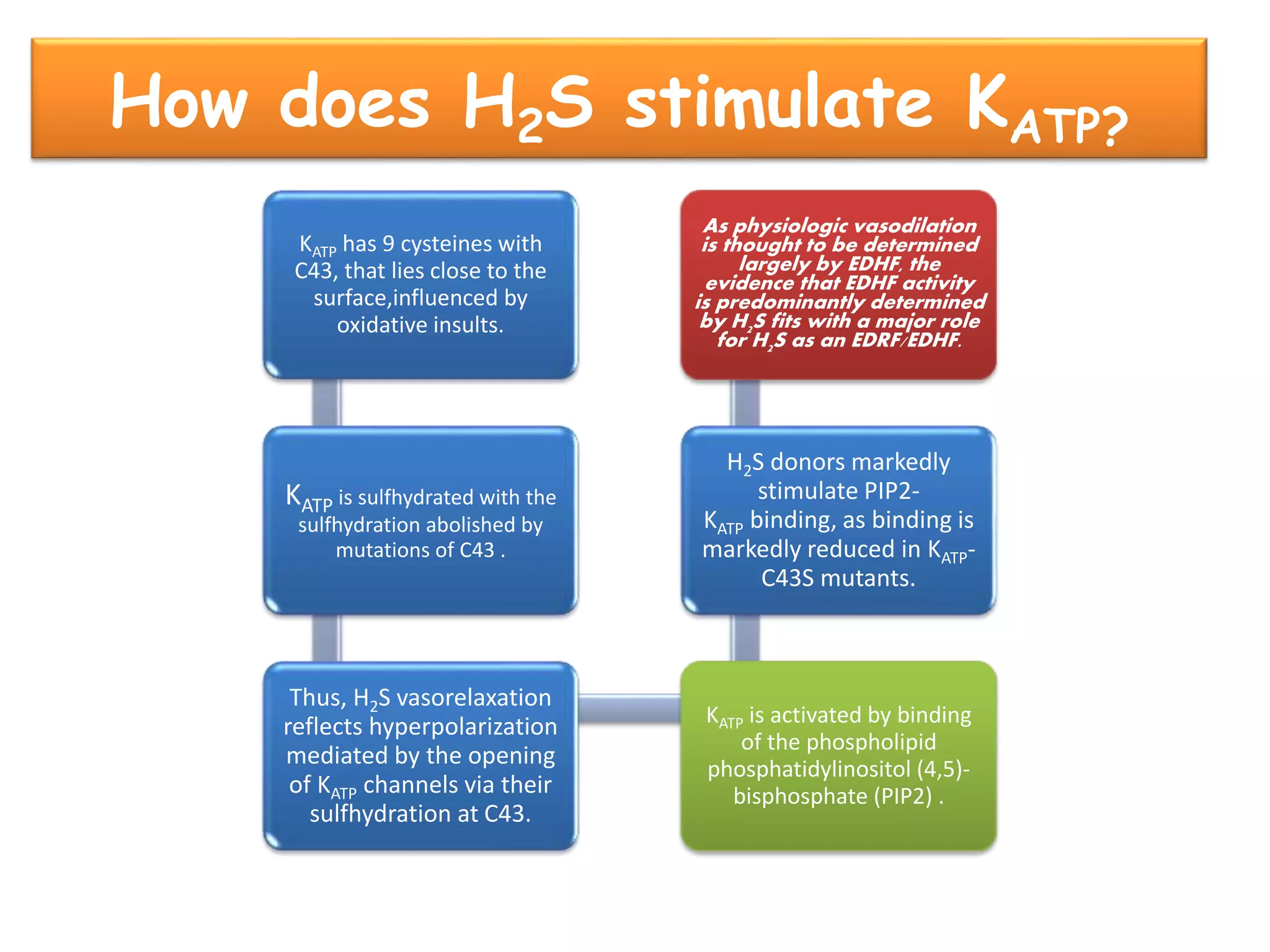
3. H2S relaxes blood vessels by opening ATP-sensitive potassium channels on endothelial cells via channel sulfhydration, causing hyperpolarization.
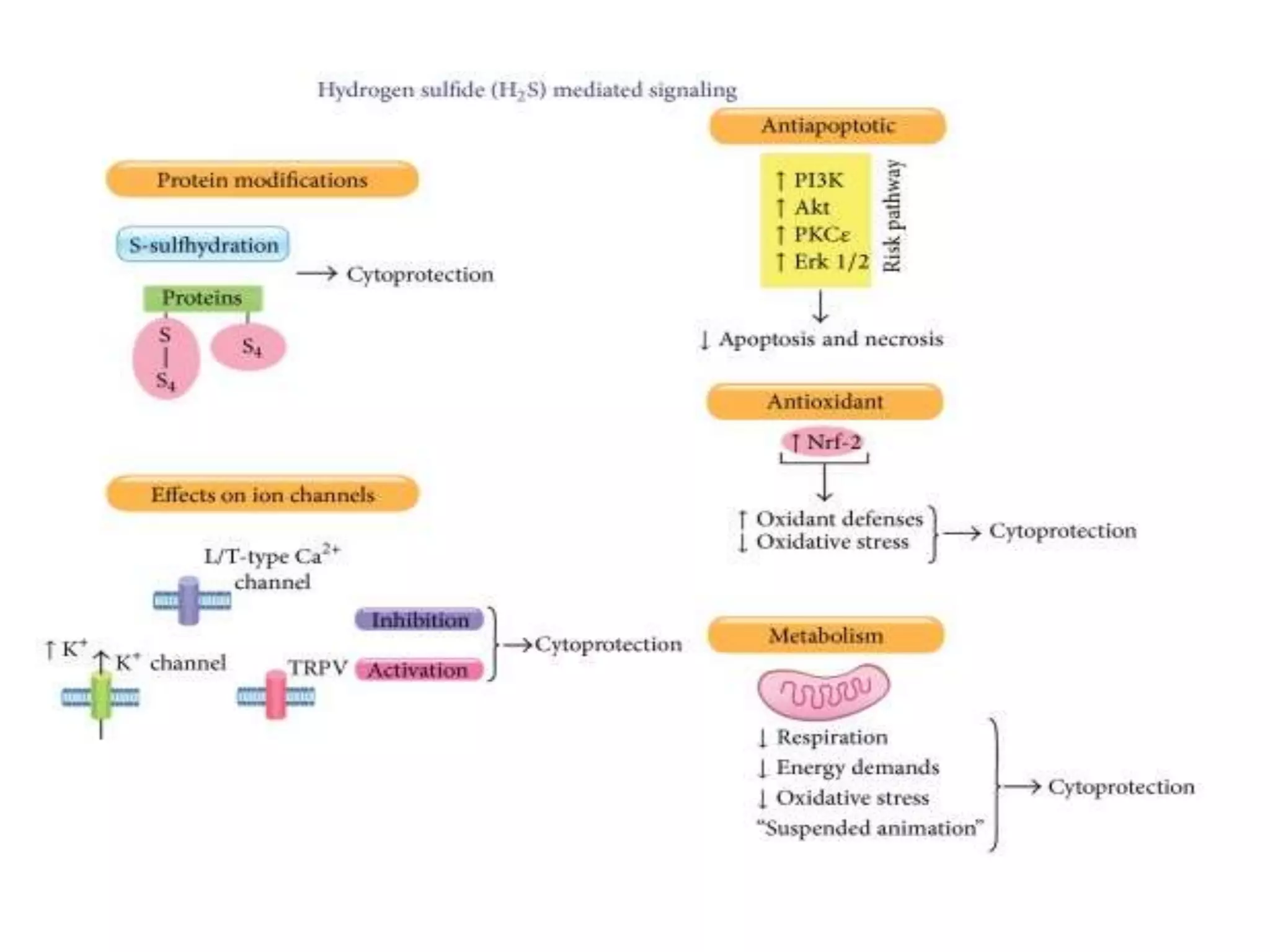
![H2S on astrocytes
• H2S donors elicit calcium waves in astrocytes and
increase intracellular levels of calcium.
• The increased [Ca]ᵢ occurs rapidly following H2S
exposure and decays slowly, whereas the
oscillations of calcium decay rapidly.
• The [Ca]ᵢ in astrocytes after H2S administration
reflects calcium entry, reduced in Ca-free media
and is associated with a direct influx of calcium
similar to that elicited by Ca ionophores.
• The type of calcium channel involved has not yet
been established.](https://image.slidesharecdn.com/hydrogensulphideasagasotransmitterloading-150119074748-conversion-gate01/75/Hydrogen-sulphide-as-a-gasotransmitter-loading-30-2048.jpg)