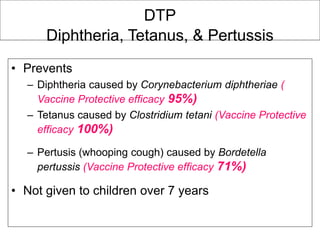
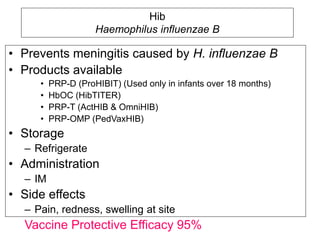
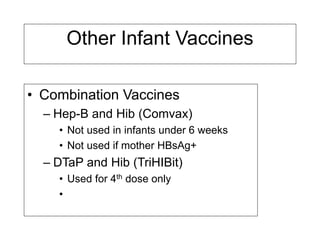
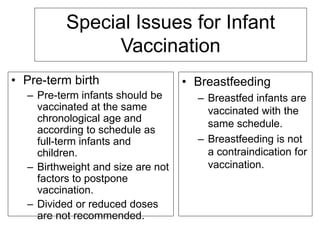
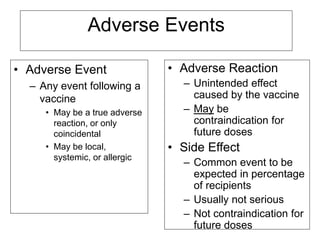
Bacterial vaccines have helped eliminate or reduce several infectious diseases. Common bacterial vaccines protect against diphtheria, tetanus, pertussis, pneumococcal disease, Hib, meningococcal meningitis, typhoid, cholera and more. Vaccines work through active immunization by vaccination or passive immunization using antibodies. Ongoing research continues to develop new vaccines and improve vaccine effectiveness.