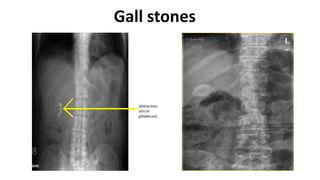
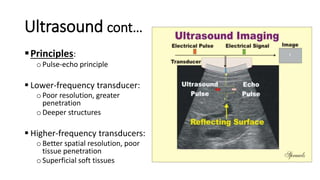
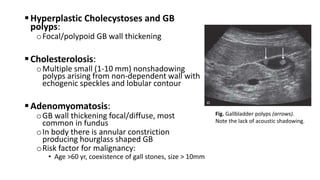
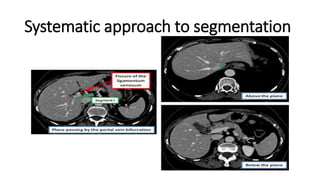
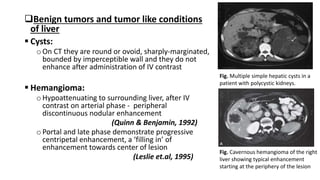
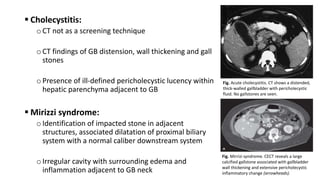
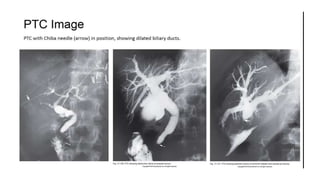
Hepatobiliary imaging uses various modalities like ultrasound, CT, MRI and others to evaluate conditions of the liver, gallbladder and bile ducts. Ultrasound is the preferred first line imaging test due to its safety, low cost, and ability to evaluate in real-time. It can detect abnormalities of the liver, gallbladder and bile ducts like cysts, masses, stones, thickening, and blockages. Characteristics seen on ultrasound help to differentiate benign from malignant conditions. Additional modalities like CT, MRI and others provide further detail when needed. Together imaging plays a key role in the evaluation and management of hepatobiliary diseases.