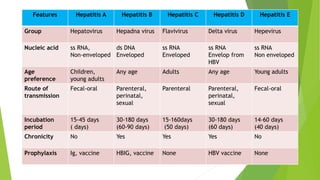
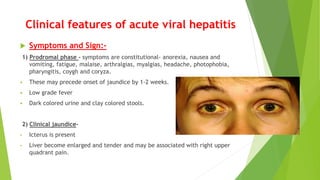
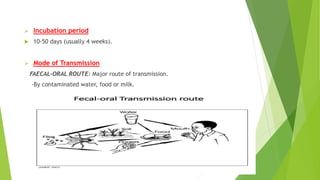
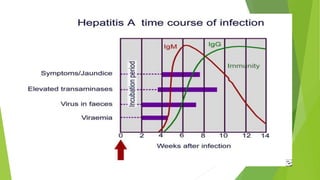
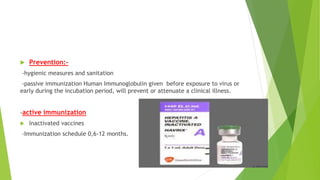
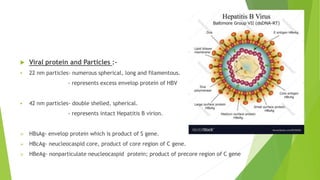
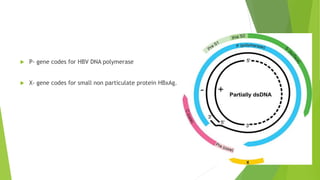
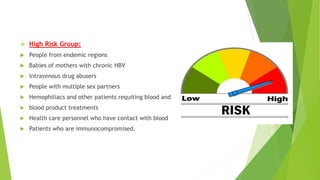
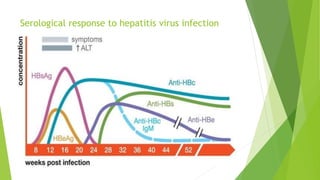
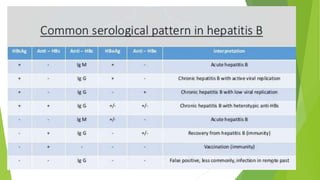
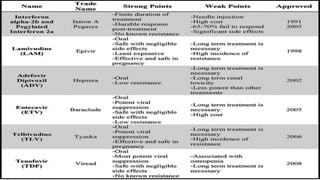
This document discusses viral hepatitis, focusing on hepatitis A, B, C. It defines viral hepatitis as inflammation of the liver caused by hepatotropic viruses. It lists the common and less common causes. It describes the key features of hepatitis A, B, C including causative agents, transmission routes, clinical presentation, investigations, management, prevention. Hepatitis A causes an acute self-limiting illness while hepatitis B and C can lead to chronic liver disease and hepatocellular carcinoma if not managed properly. Prevention involves vaccination and hygienic measures.