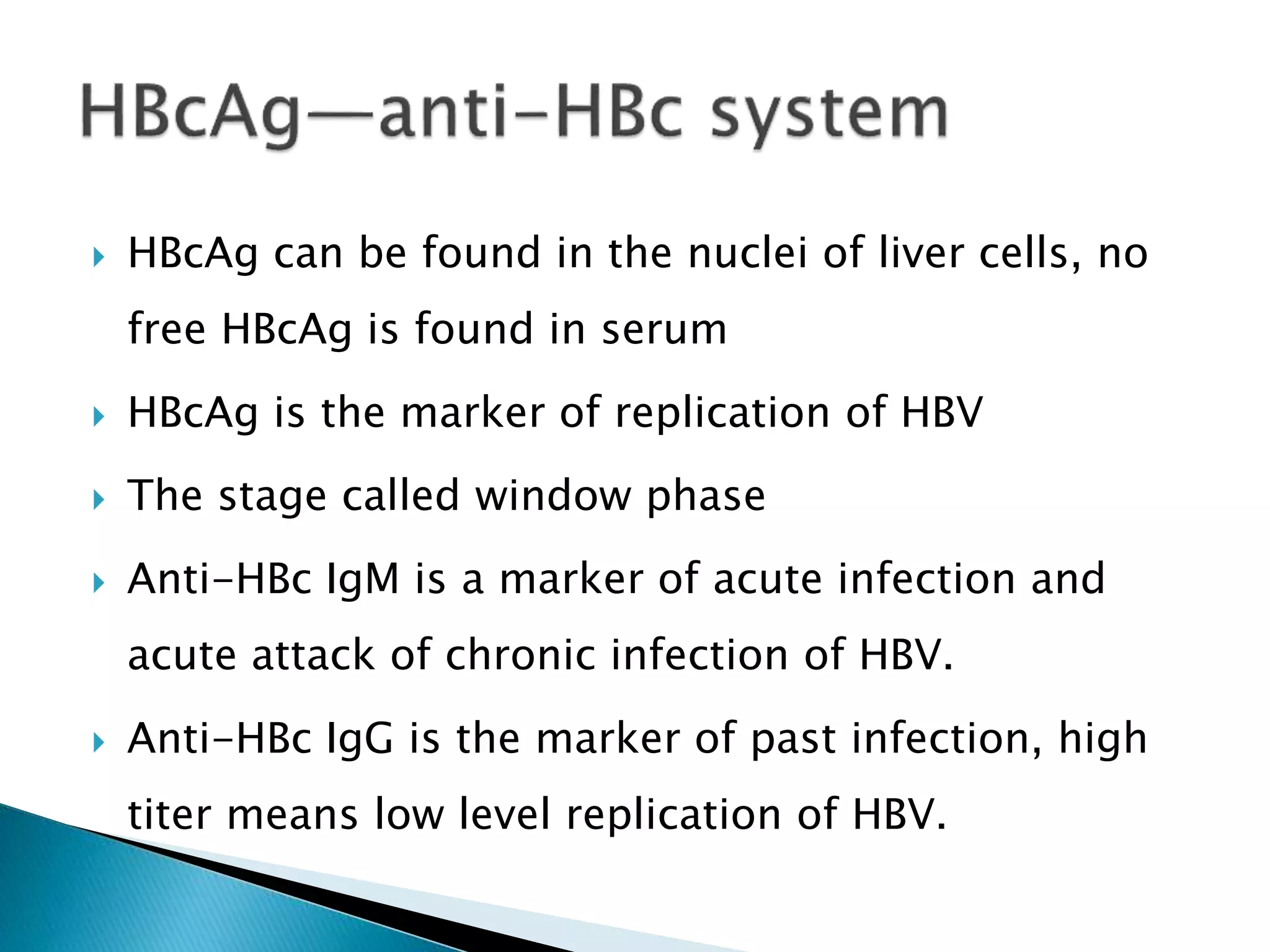
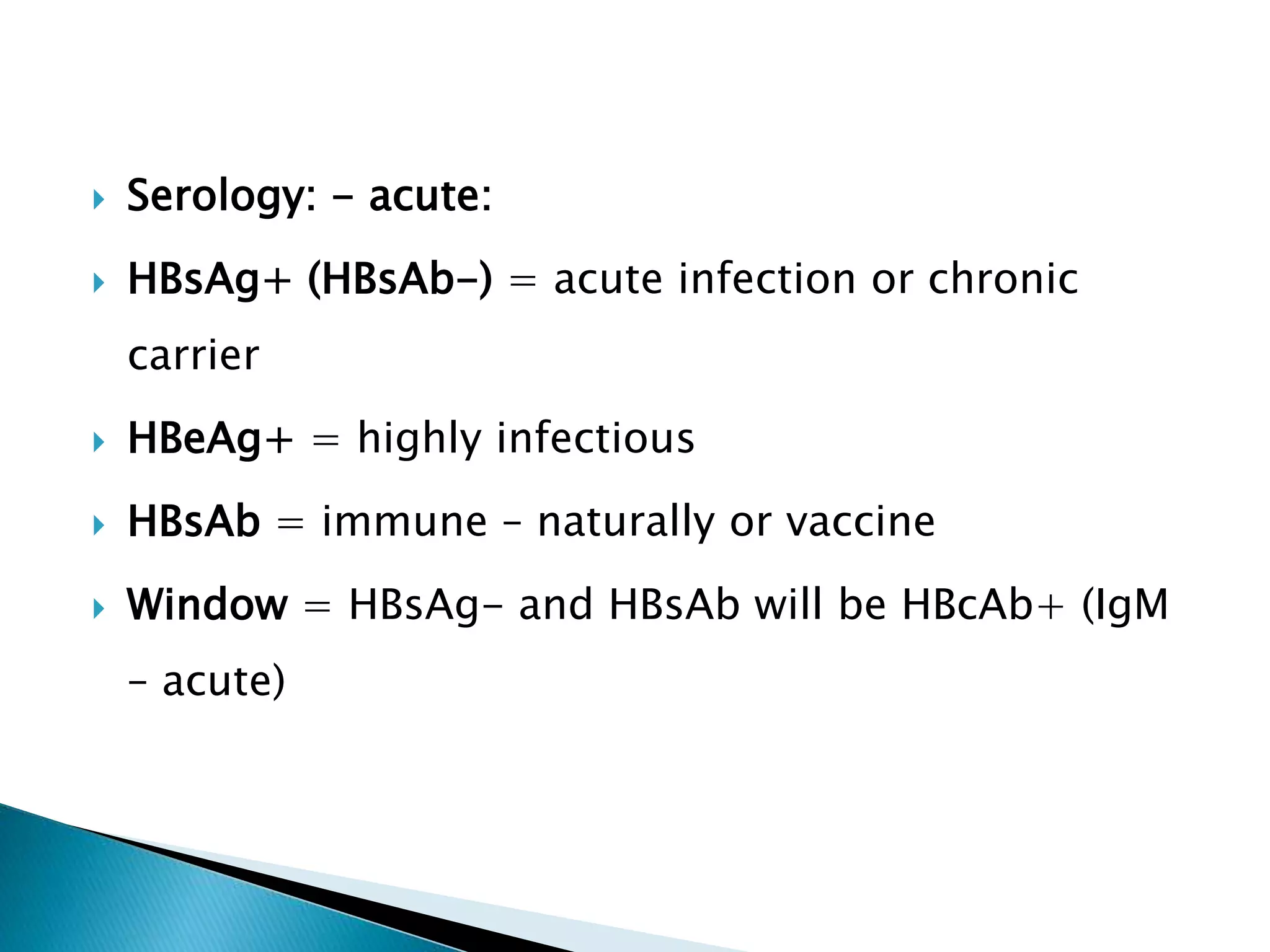
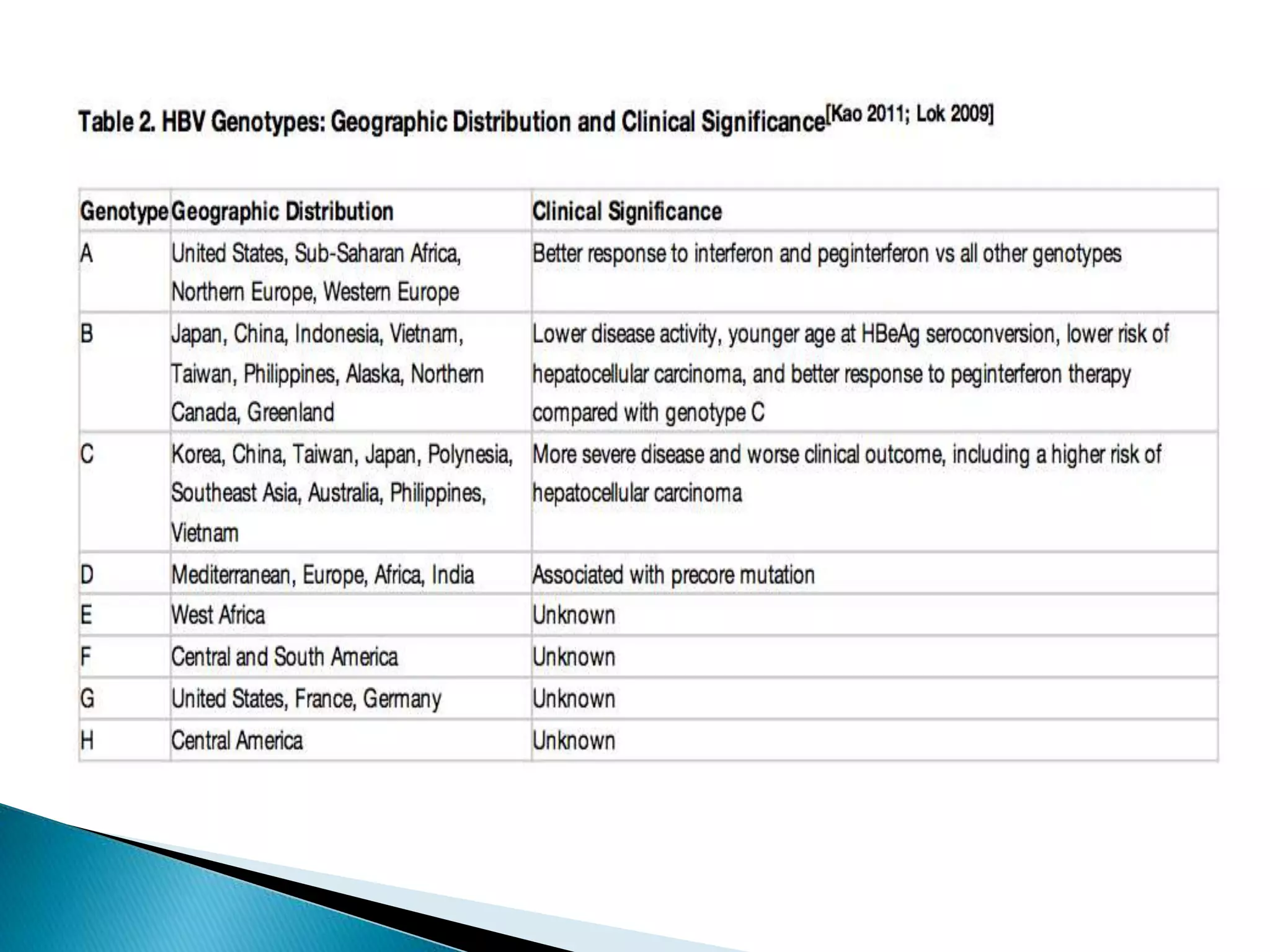
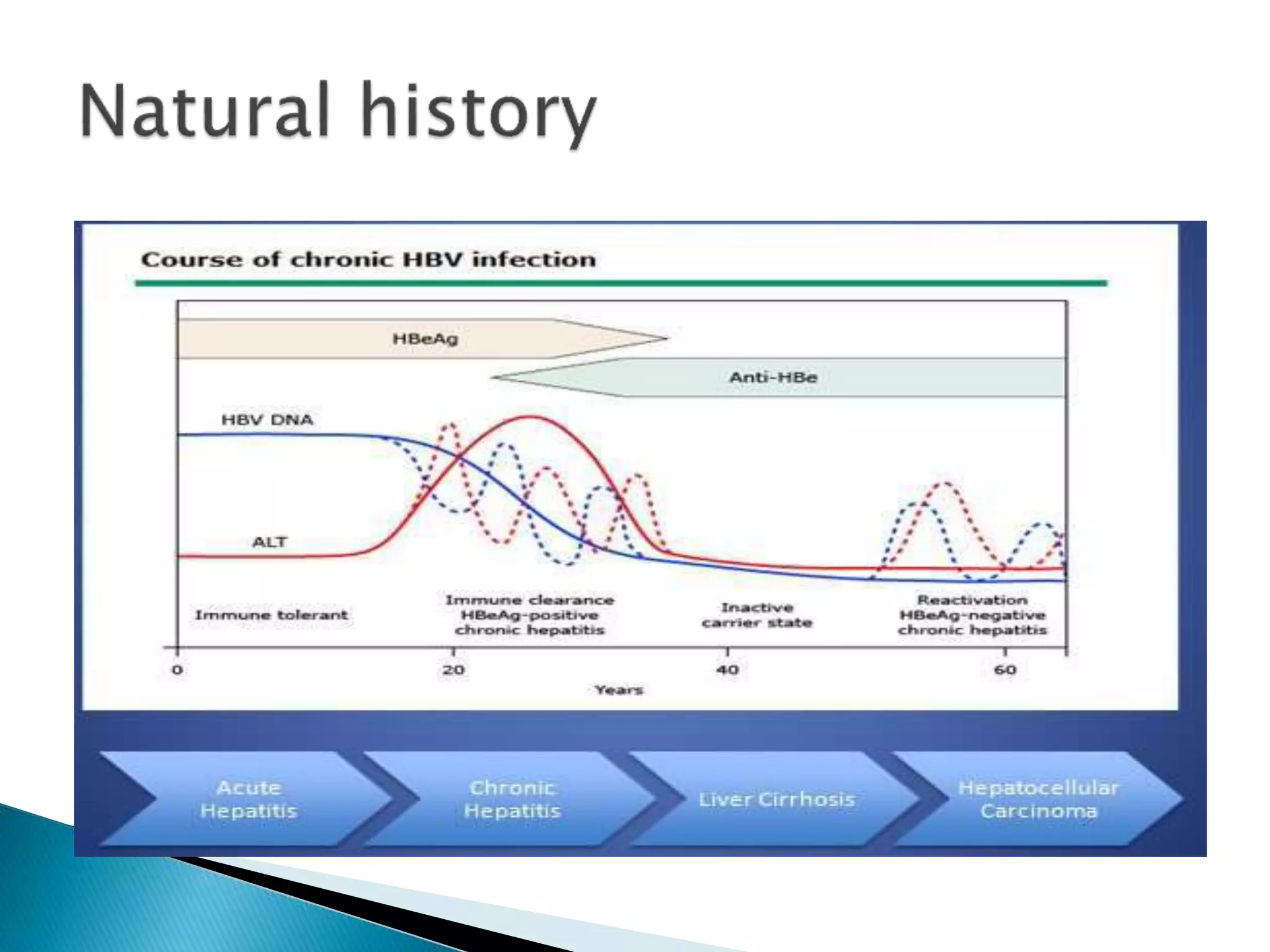
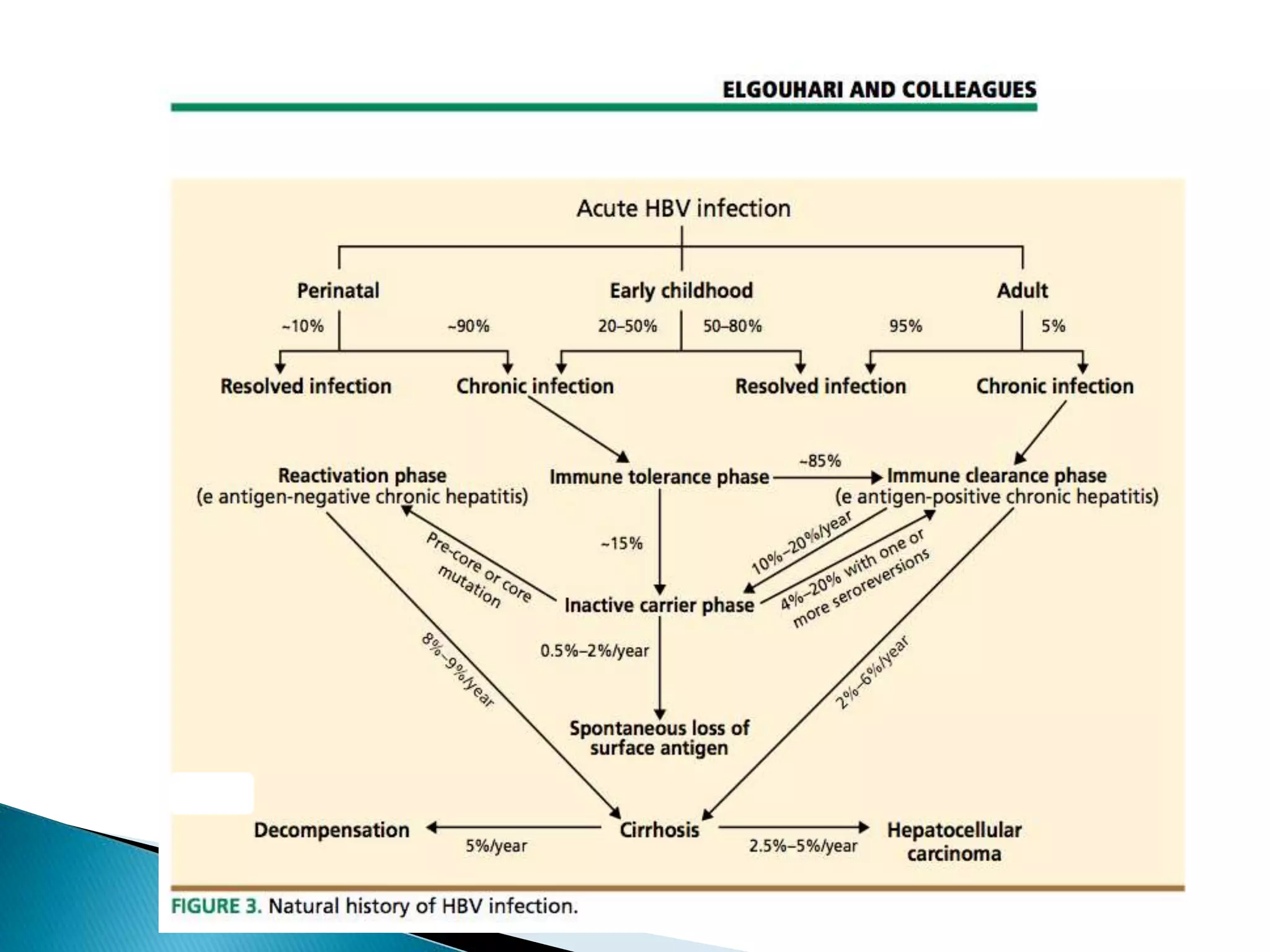
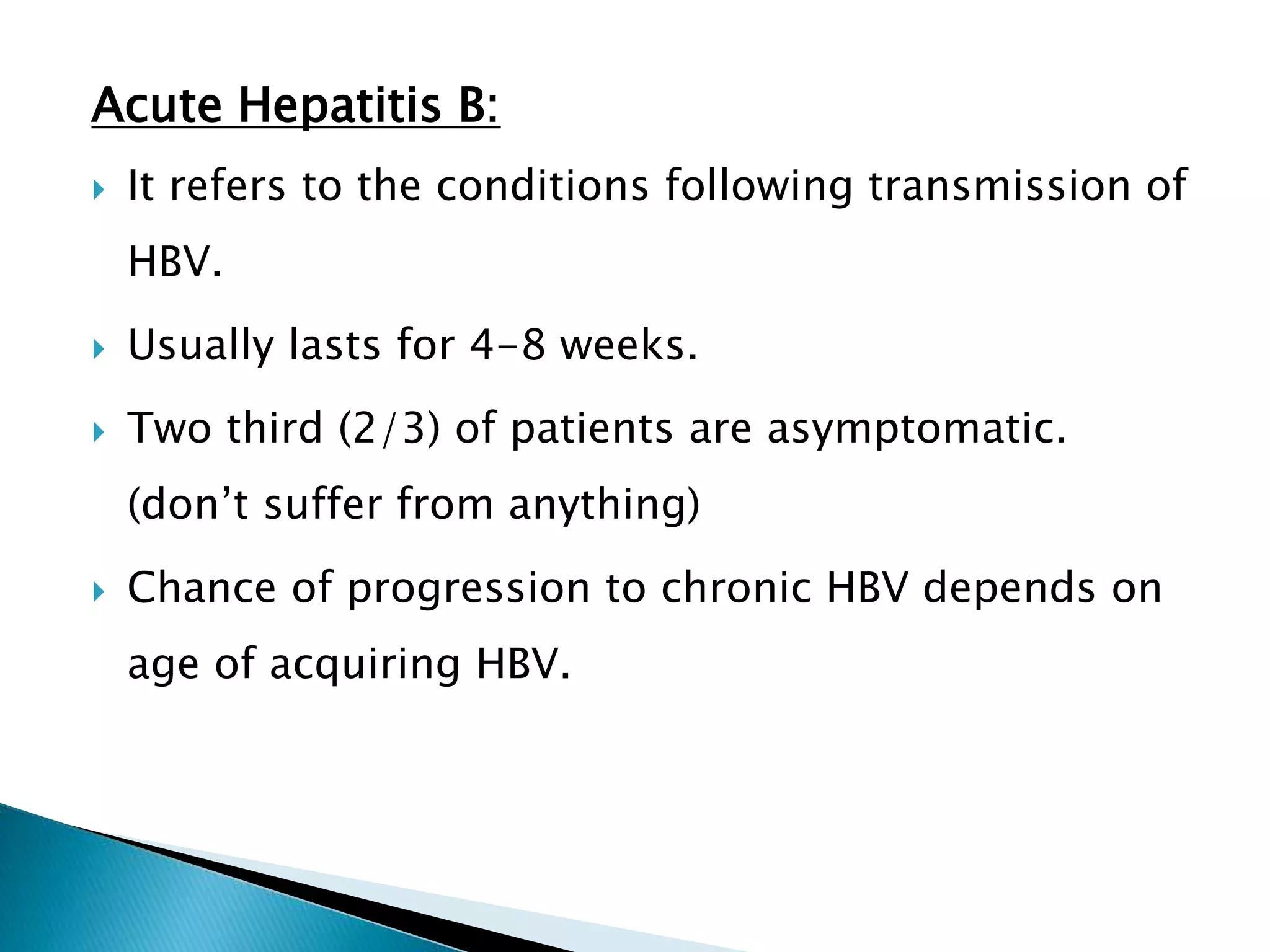
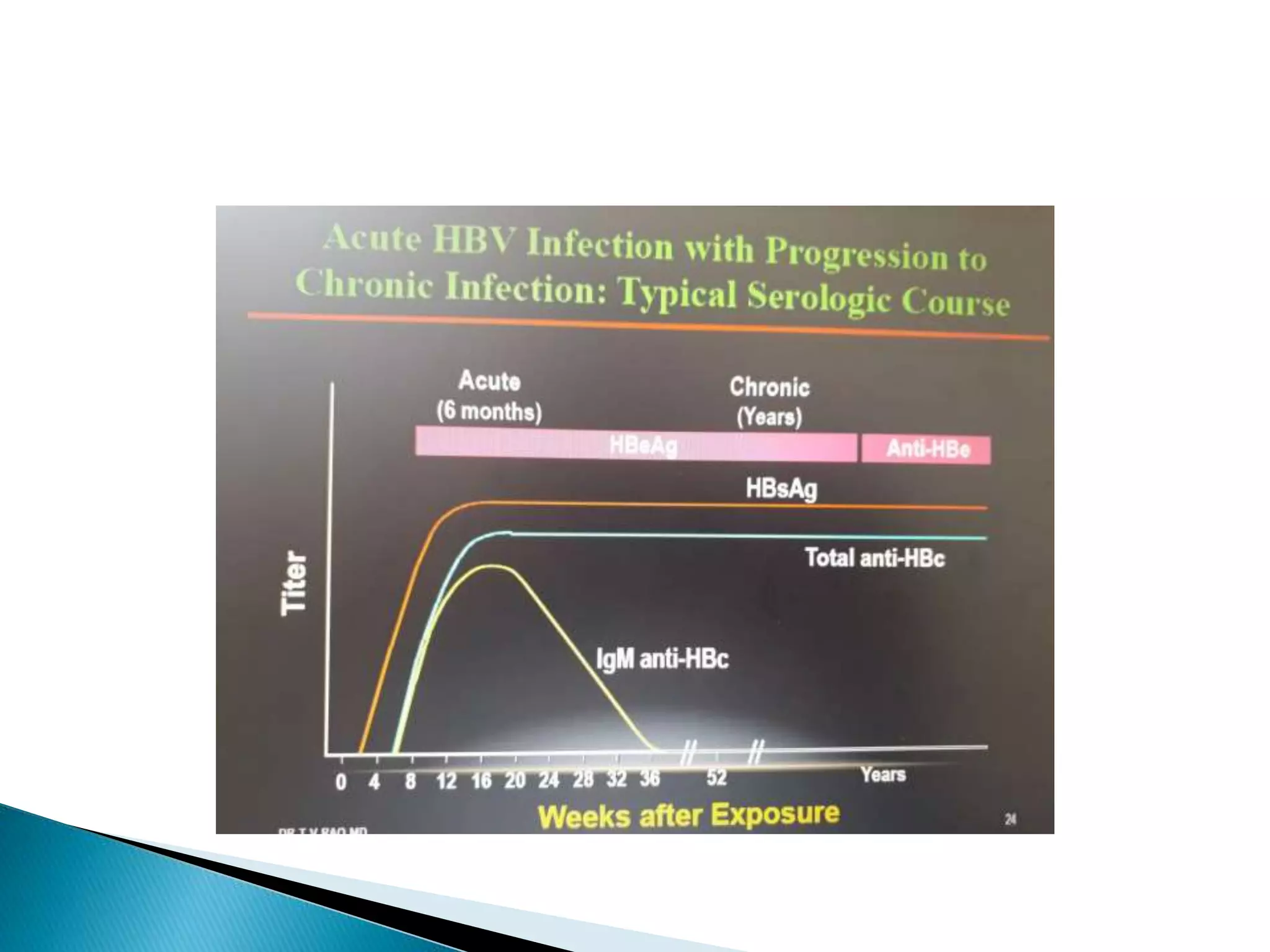
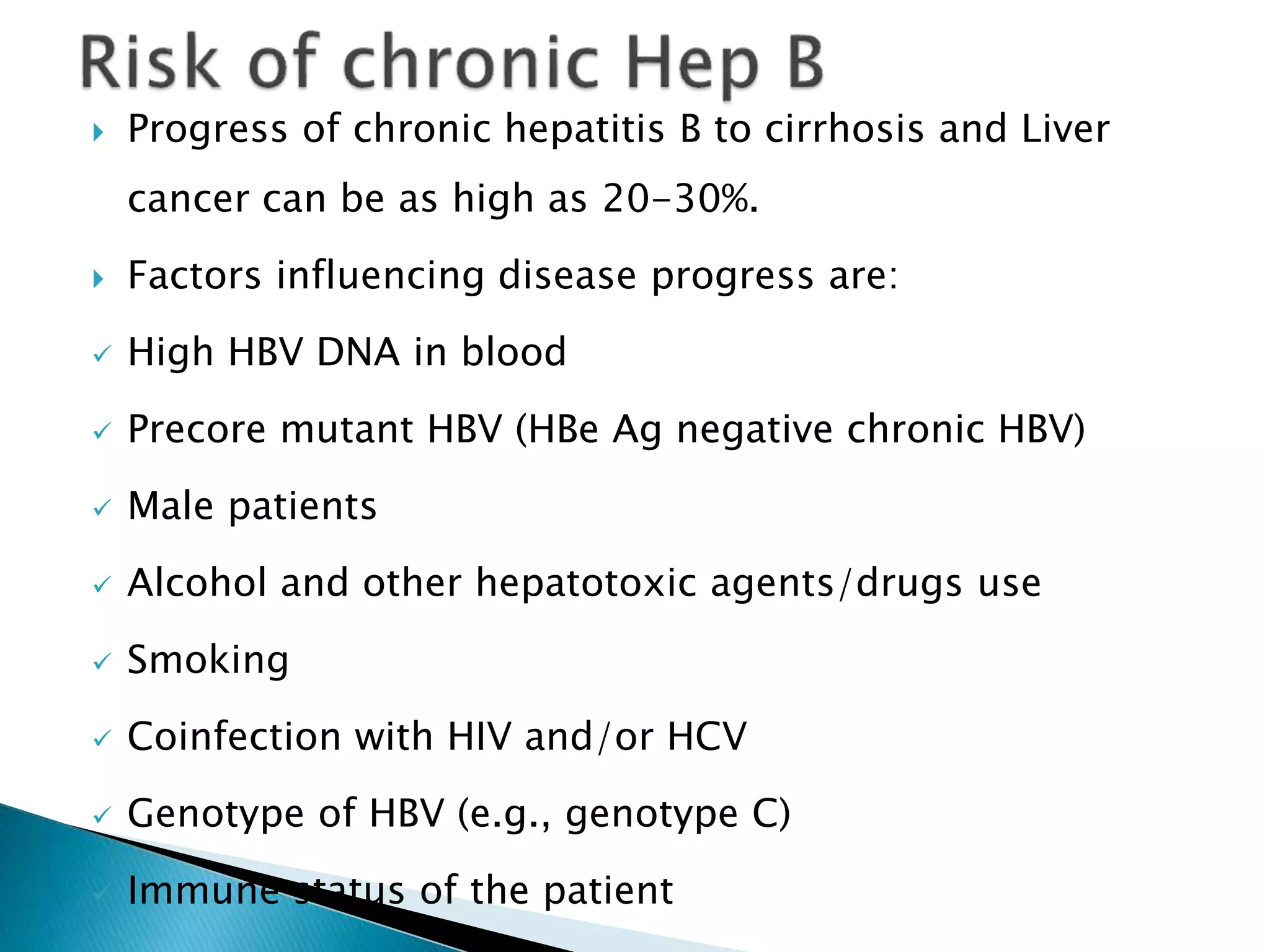
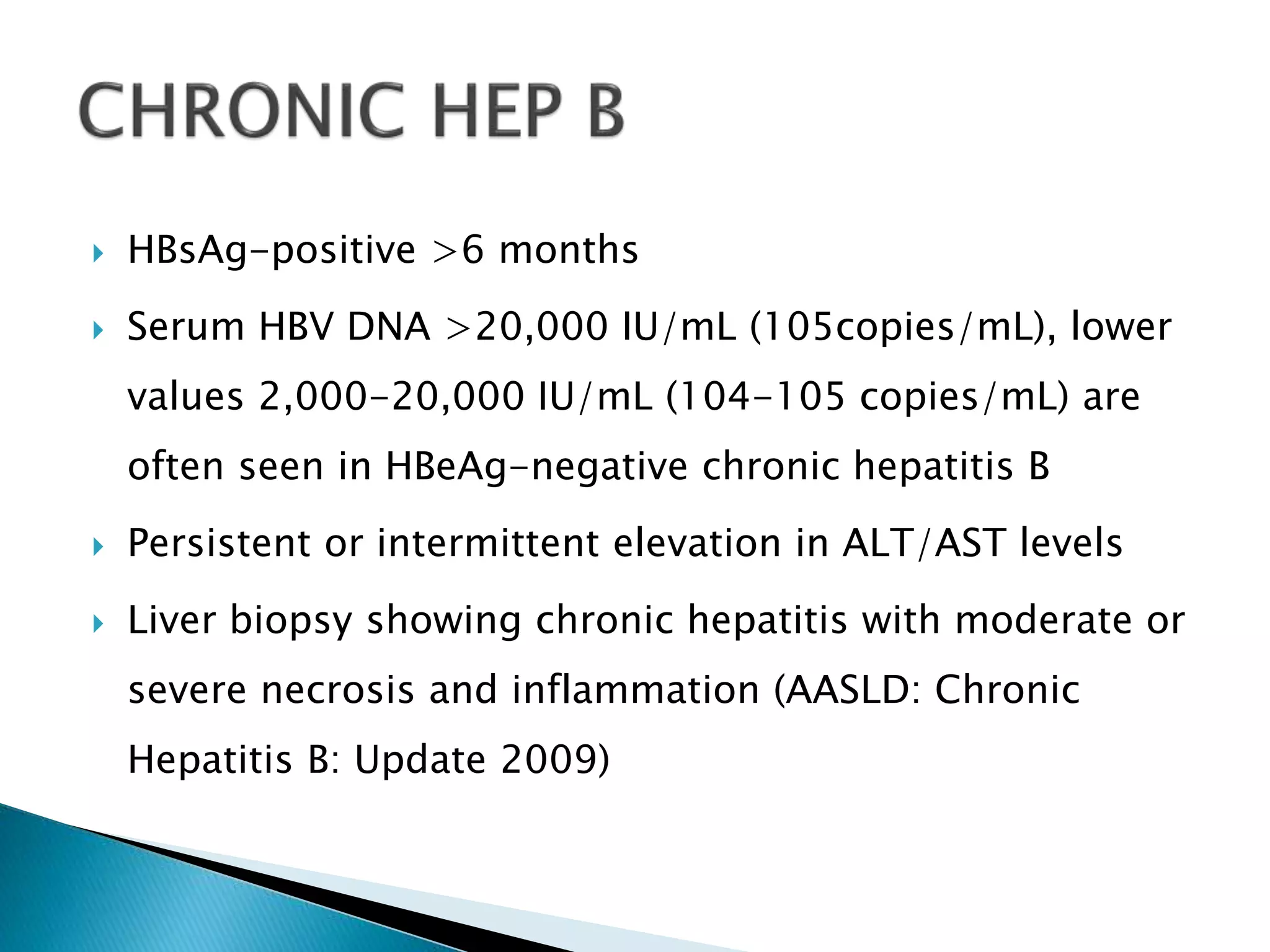
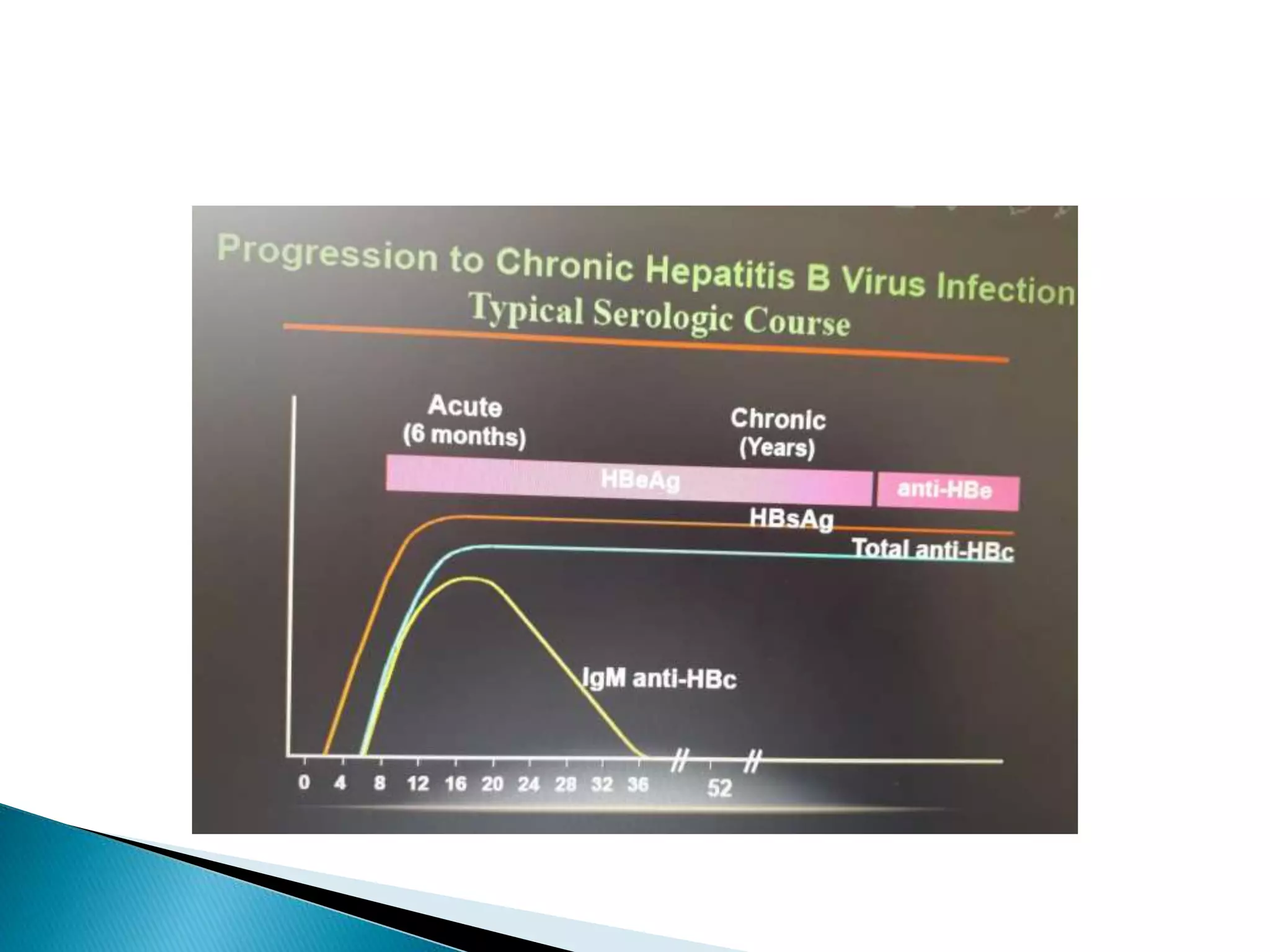
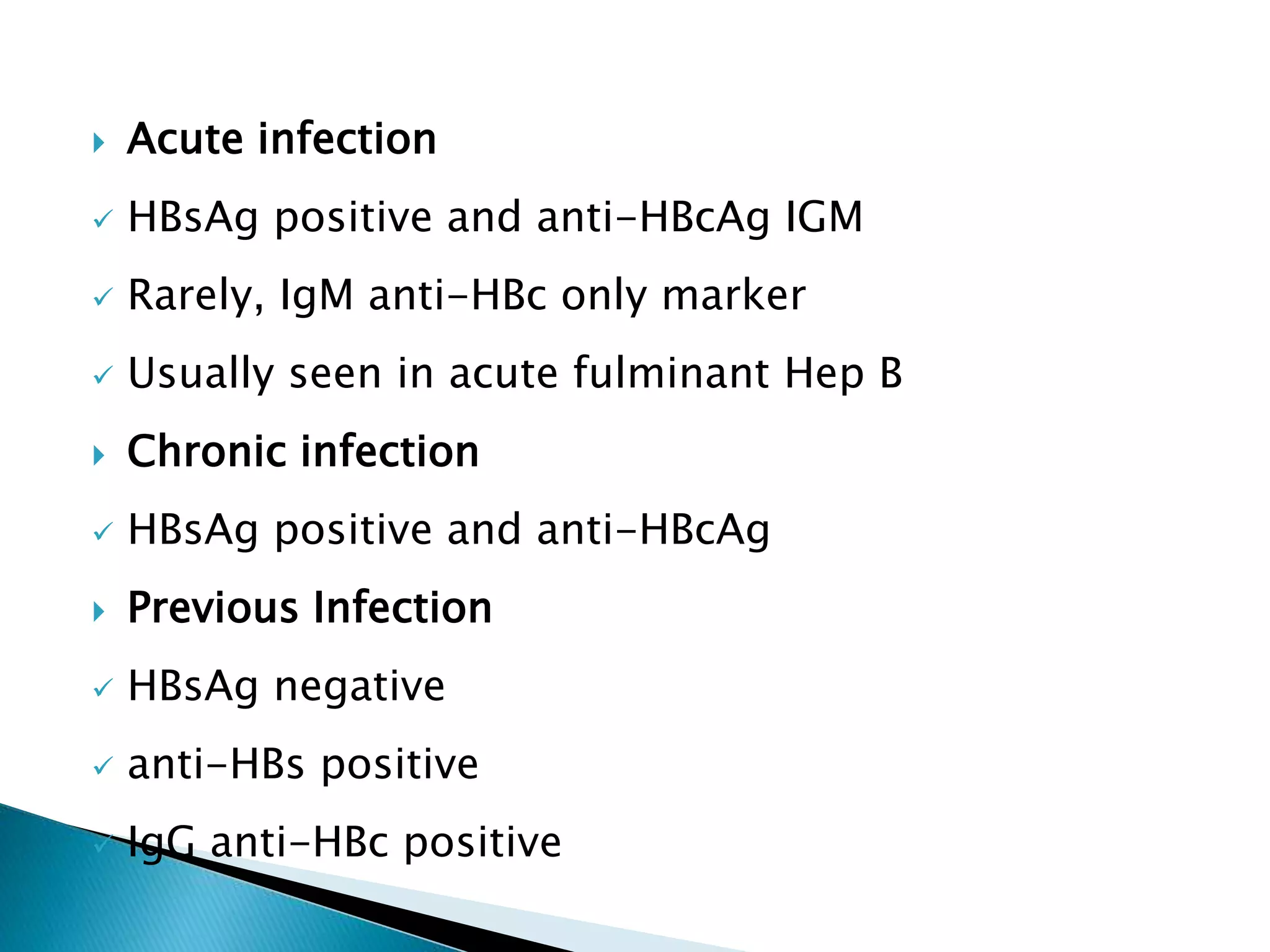
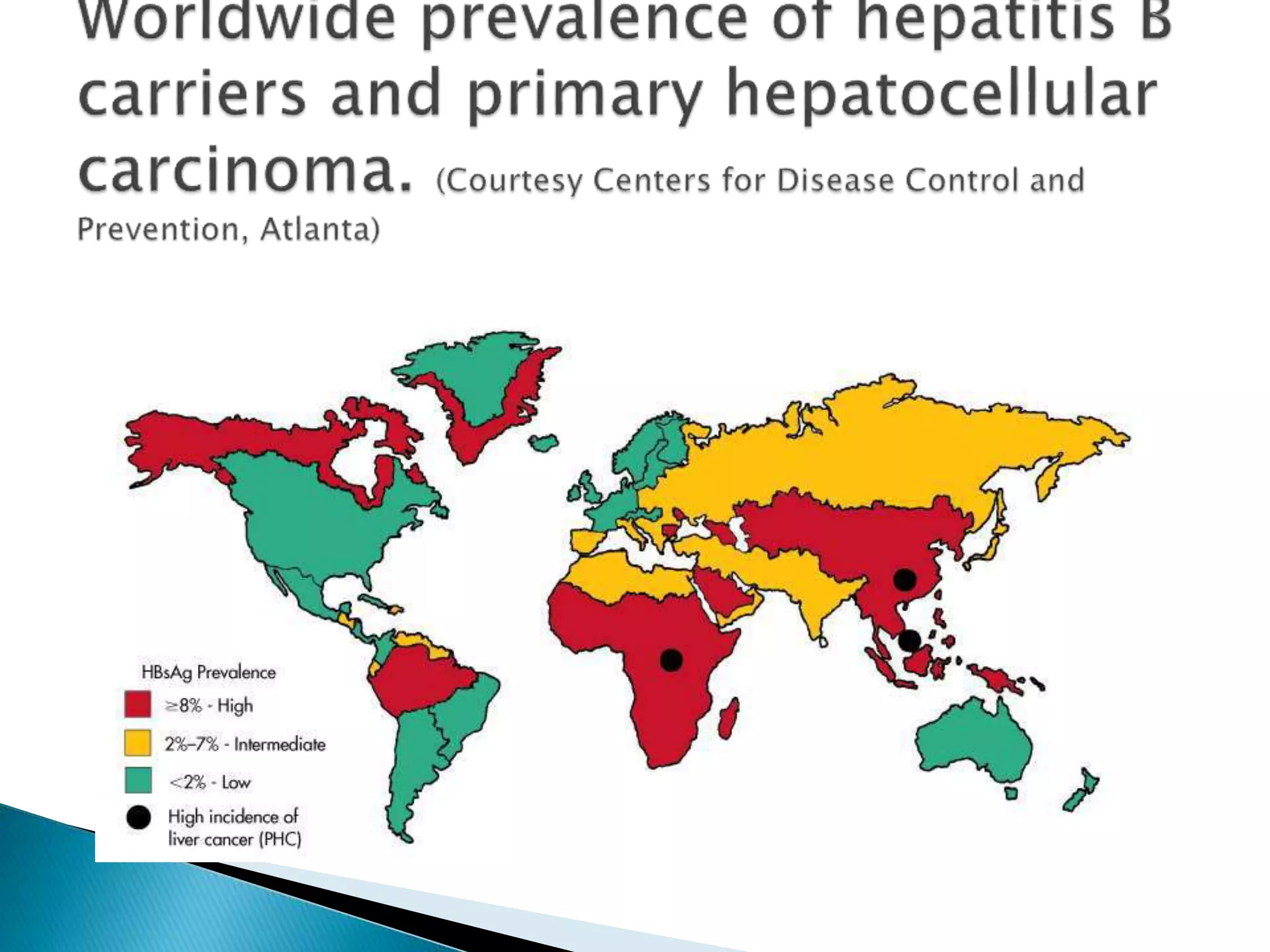
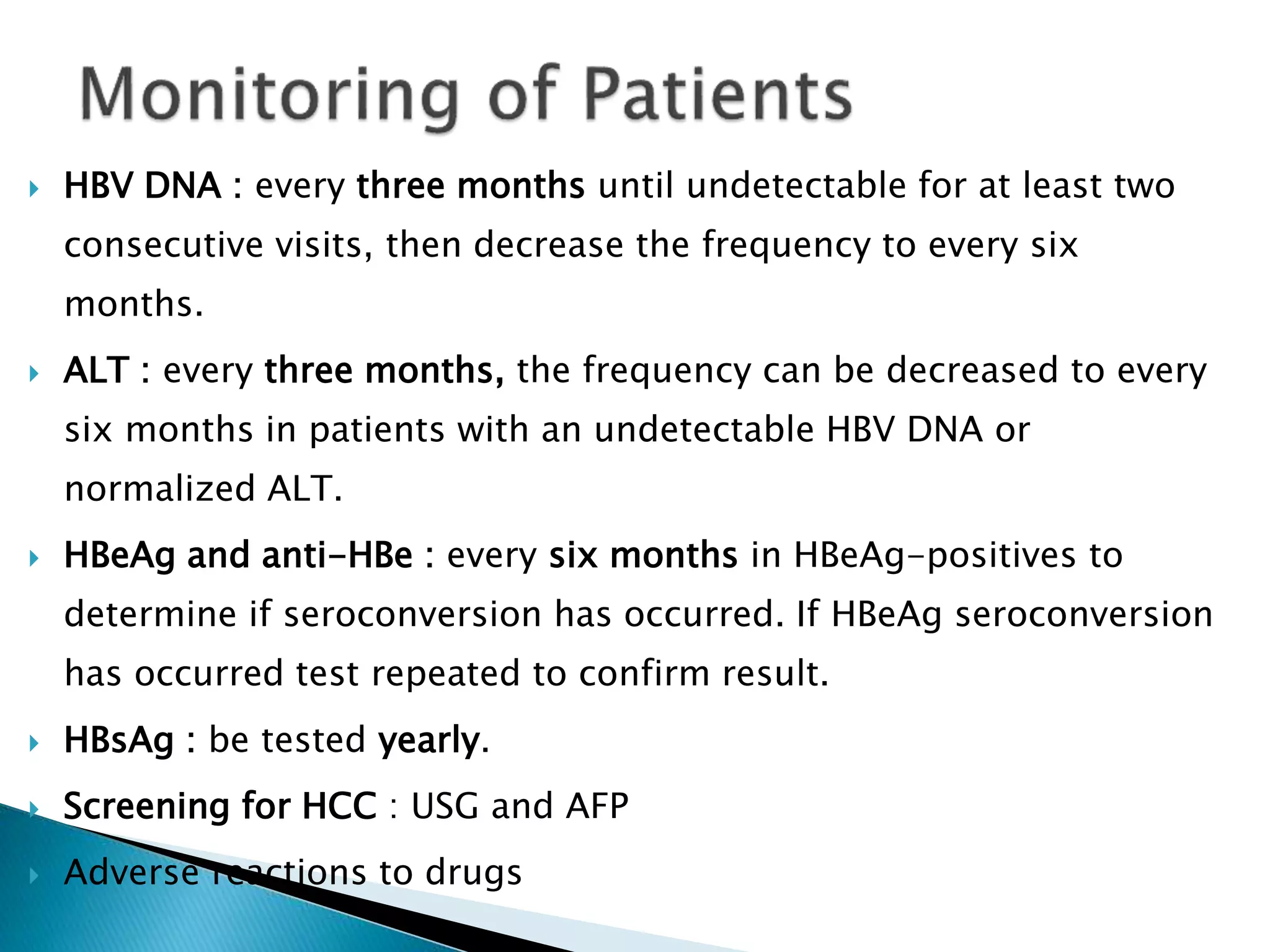
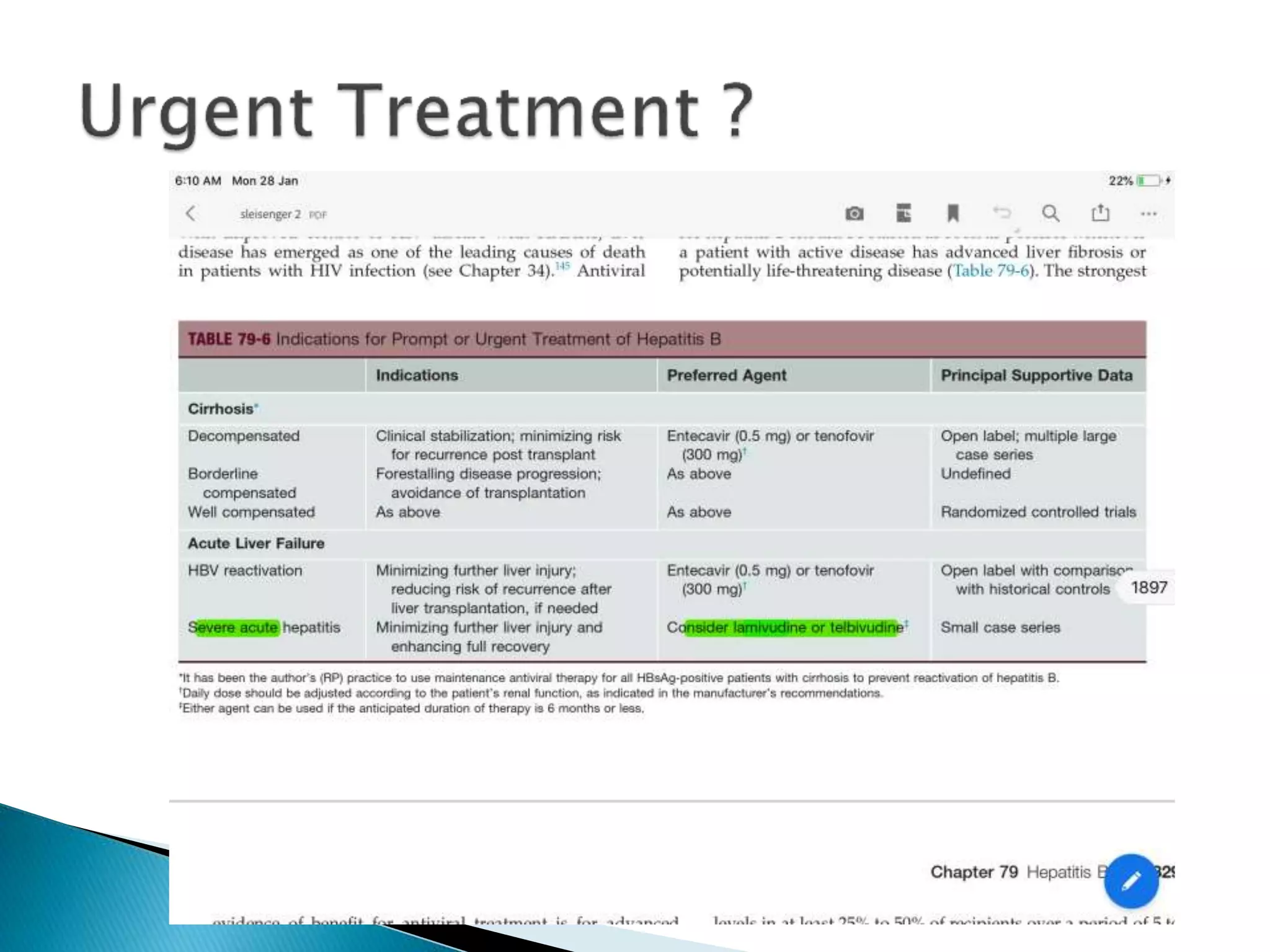
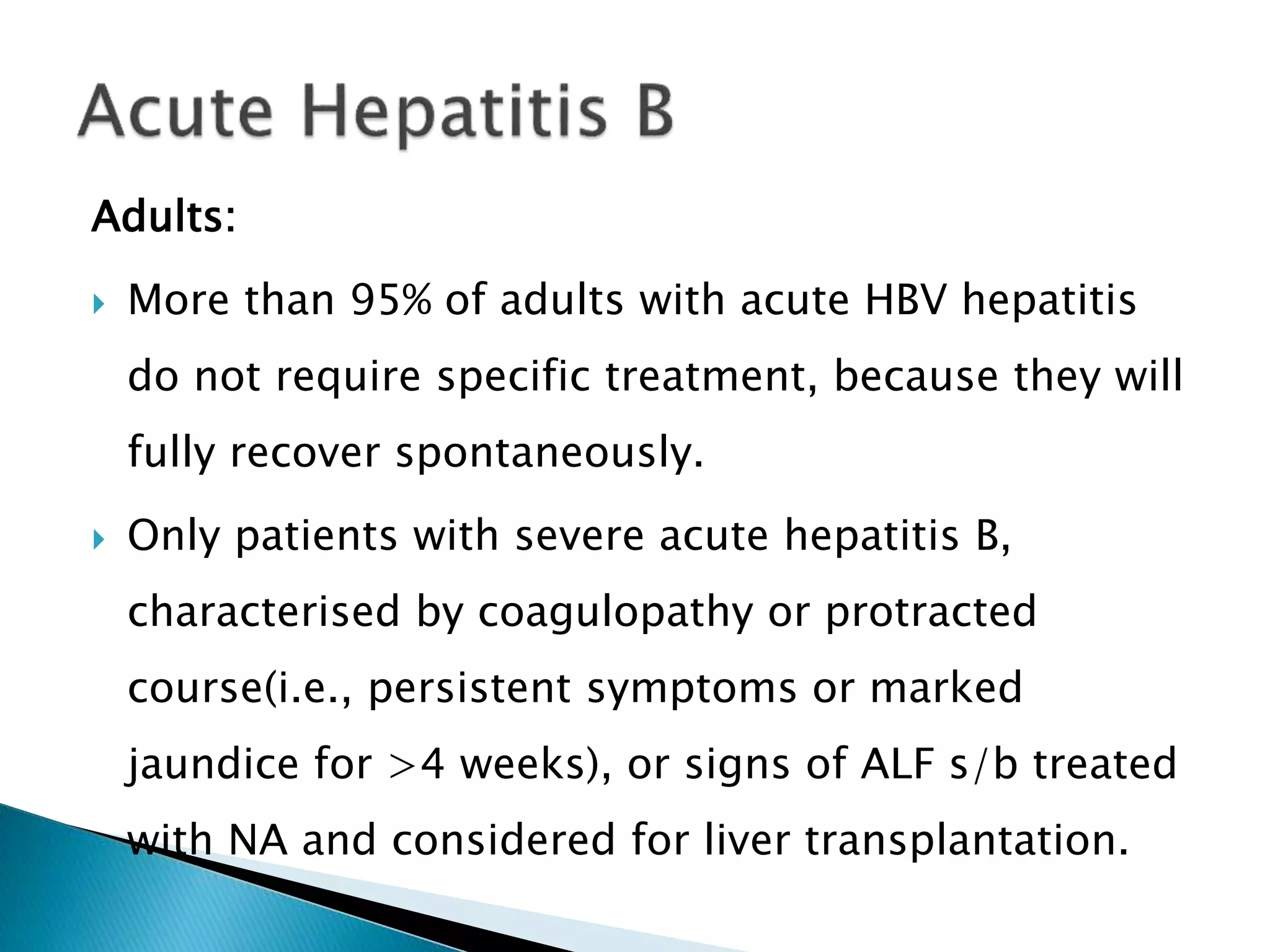
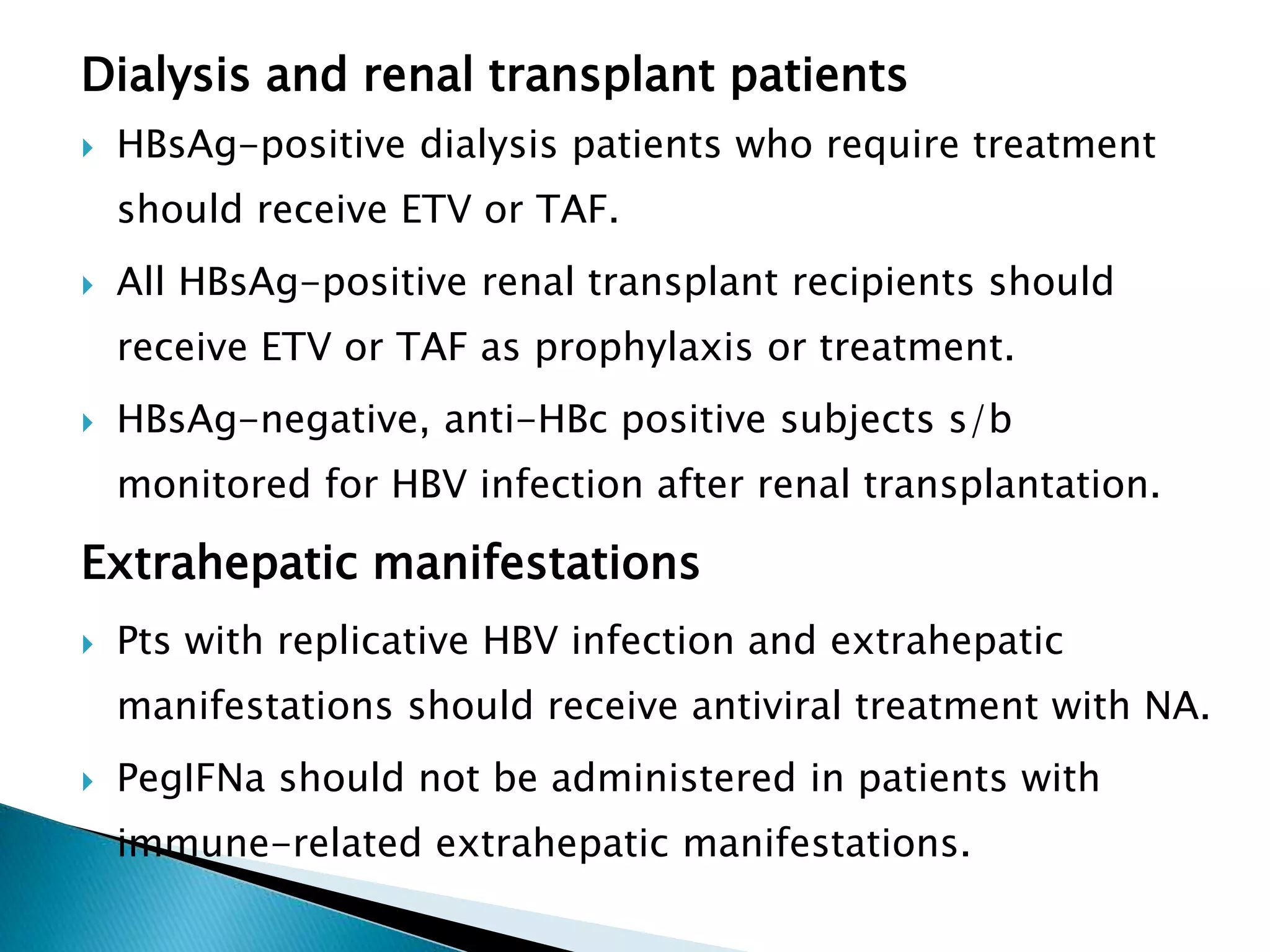
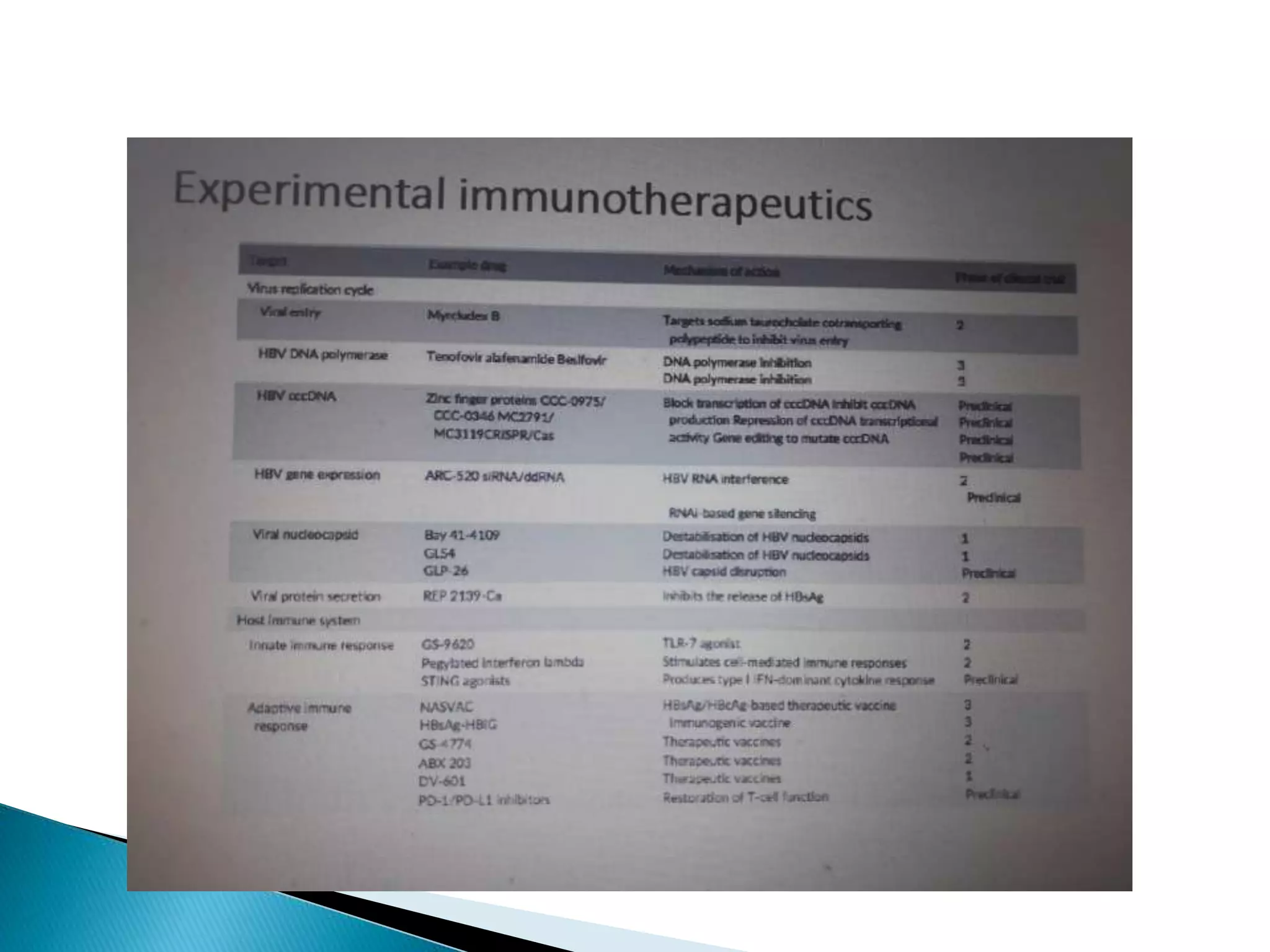
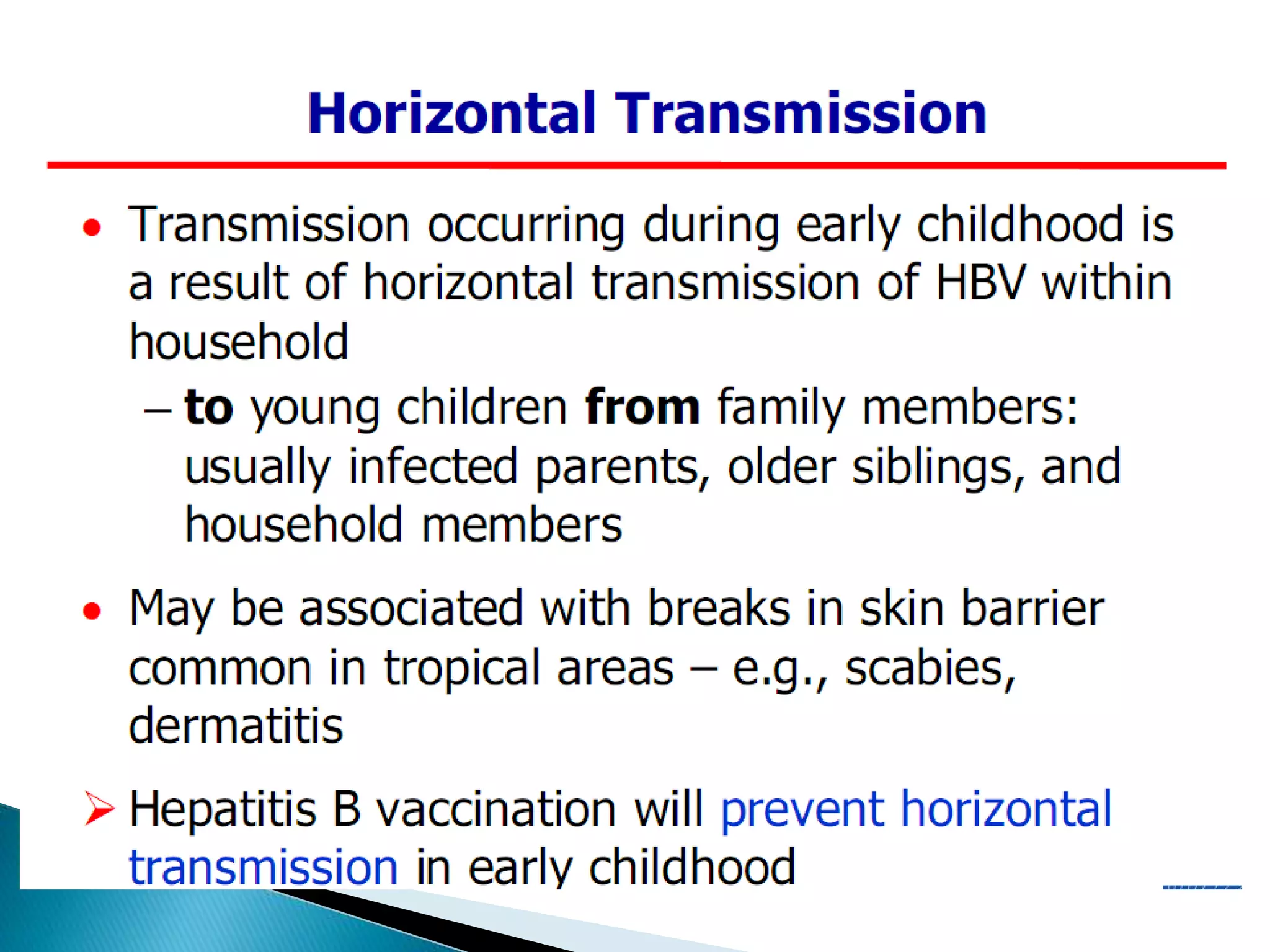
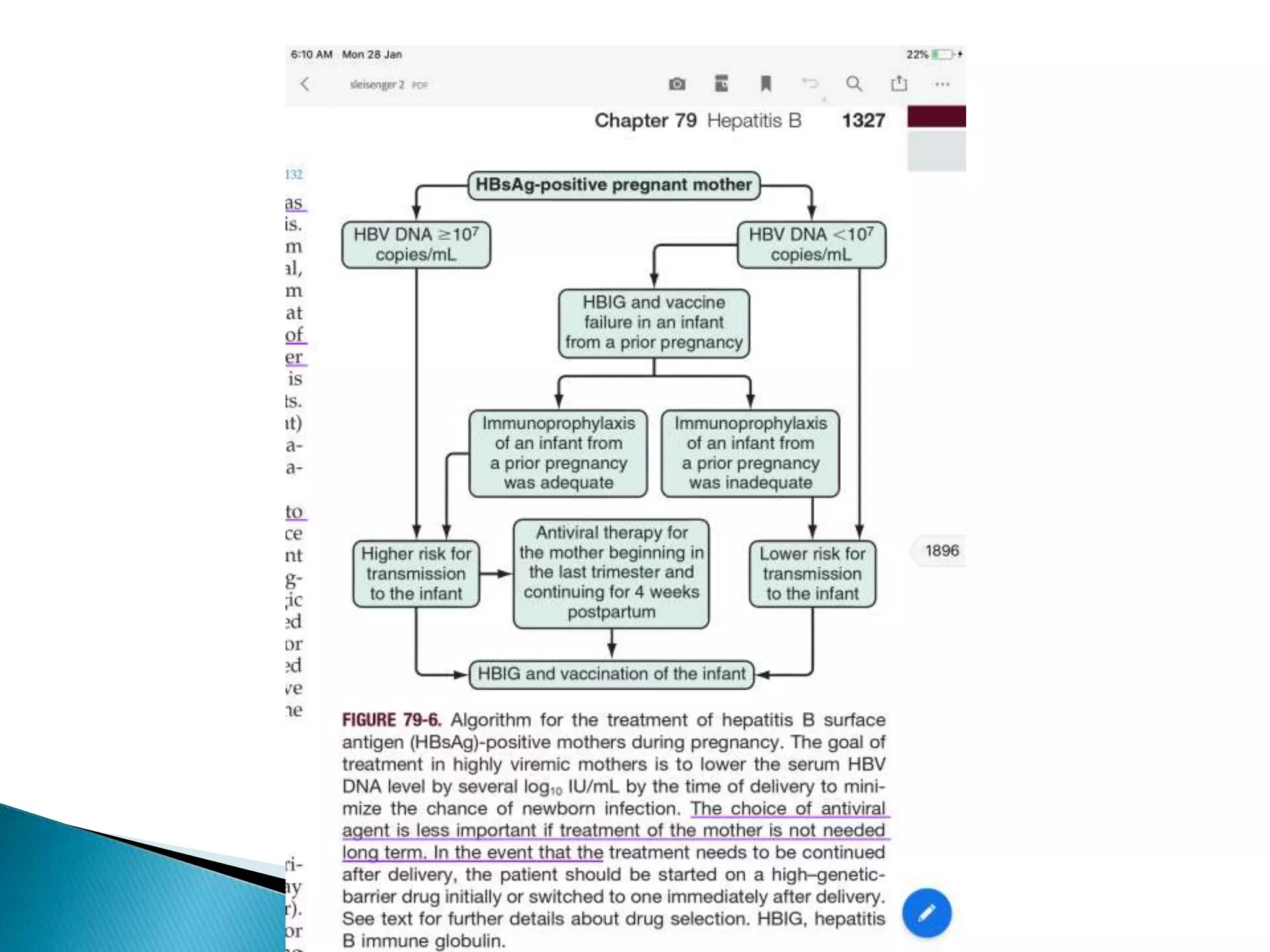
This document discusses the history, virology, transmission, diagnosis, and treatment of hepatitis B virus (HBV) infection over several pages. It notes that HBV was discovered in 1965 and its virus particle identified in 1970. HBV is transmitted through blood and bodily fluids and can be prevented through vaccination. Diagnosis involves testing for various hepatitis B antigens and antibodies. Treatment goals are to prevent disease progression and complications through suppression of HBV replication. First-line treatments include interferons and nucleoside analogues administered for at least 12 months after HBeAg seroconversion or lifelong in cirrhosis.