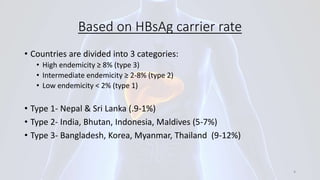
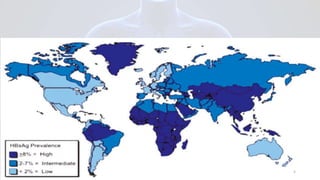
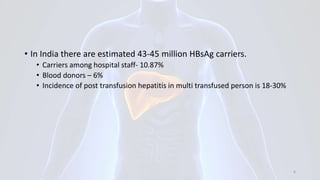
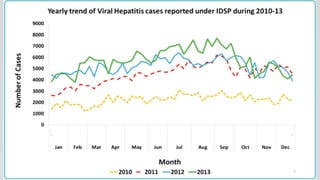
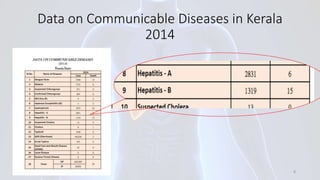
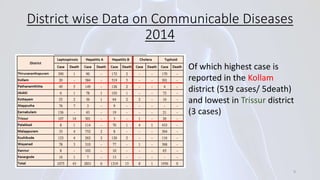
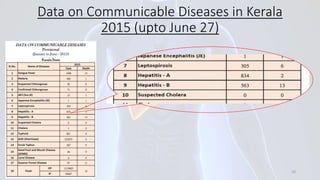
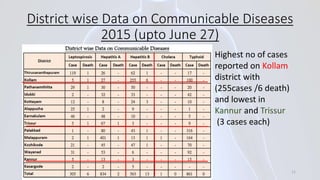
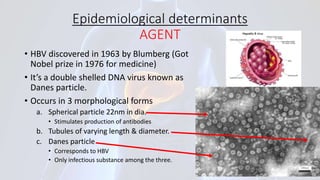
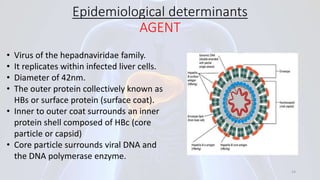
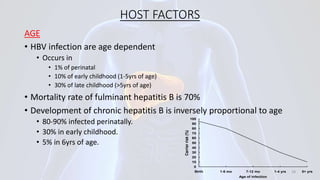
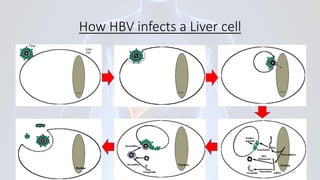
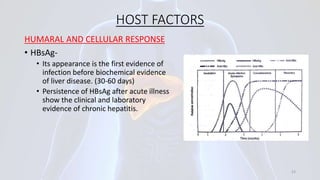
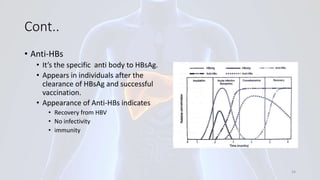
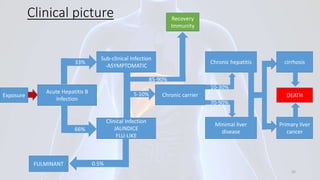
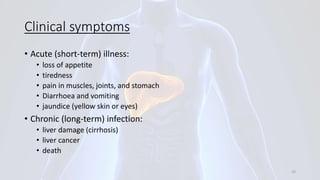
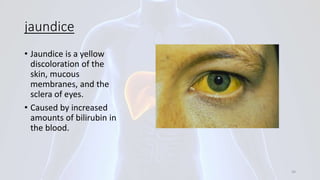
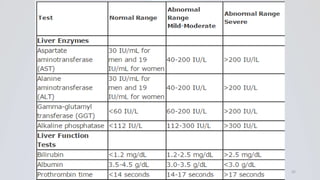
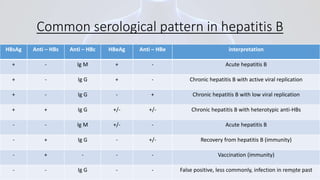
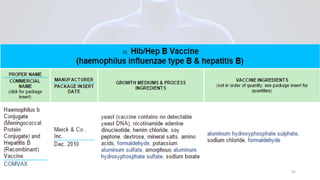
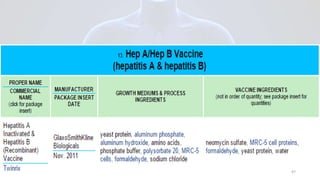
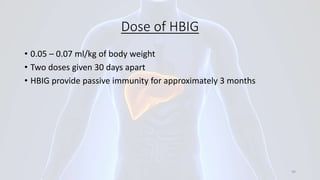
Hepatitis B is a viral infection that affects the liver. It is caused by the hepatitis B virus and is transmitted through contact with infected blood or bodily fluids. The virus can cause both acute and chronic infections. Chronic infections may lead to serious health issues like liver damage, cirrhosis, and liver cancer. Hepatitis B is a major global health problem, with millions of people infected worldwide. Vaccination is the most effective way to prevent hepatitis B infection.