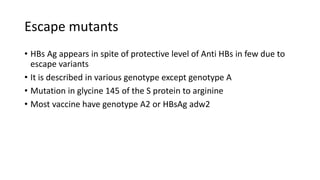
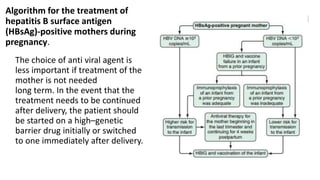
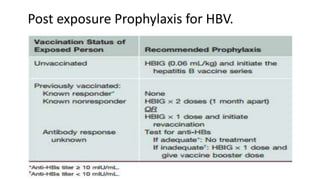
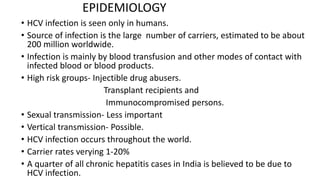
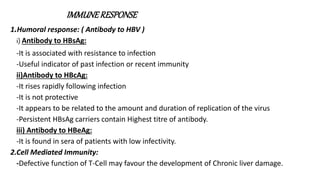
Hepatitis B virus was discovered in the 1980s through outbreak investigations. It is a DNA virus belonging to the Hepadnaviridae family. About 2 billion people have been infected worldwide, with 400 million having chronic infections. It is transmitted through bodily fluids and from mother to child.
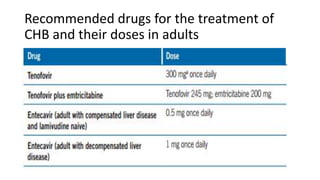
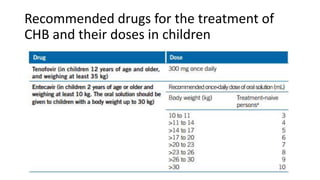
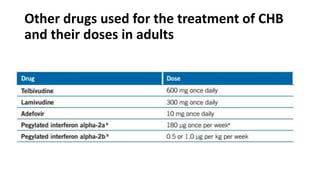
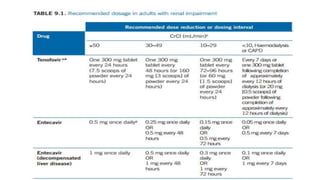
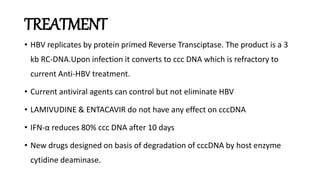
Acute infection presents with non-specific symptoms like fatigue and jaundice. Chronic infection can lead to liver damage and cancer. Diagnosis involves detecting viral antigens and antibodies. While treatment cannot eliminate the virus, antivirals can suppress it. The WHO recommends treatment for those with significant liver disease or high risk of progression. Treatment aims to improve outcomes of this serious public health problem.





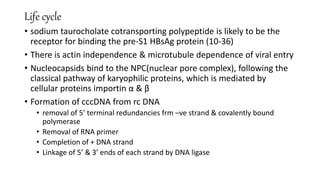
![The Genome
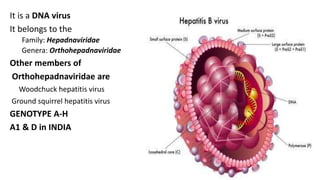
• HBV is a small (3.2-kilobase [kb]) virus with a DNA genome that has a relaxed,
circular, partially double-stranded configuration.
• The genome is composed of 4 open reading frames
(ORFs) and has a compact design in which several genes overlap and use the
same DNA to encode different viral proteins.
• The 4 viral genes components include the core, surface,
X, and polymerase genes.
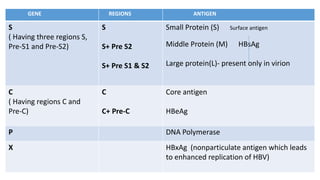
• The core gene encodes the core nucleocapsid protein, which is important in viral
packaging and production of HBeAg.
• The surface gene encodes the pre-S1, pre-S2, and S proteins (comprising the
large [L], middle [M], and small [S] surface proteins).
• The X gene encodes the X protein, which has transactivating properties and may
be important in hepatic carcinogenesis,
•The polymerase gene has a large ORF ( ≈800 AA). It encodes a large protein with functions that are critical for packaging and DNA replication
(including priming, RNA- and DNA-dependent DNA polymerase, and RNase activities).](https://image.slidesharecdn.com/seminarhepbjoy-150820130939-lva1-app6892/85/hepatitis-B-VIRUS-9-320.jpg)
















![NON-INVASIVE ASSESSMENT OF LIVER DISEASE STAGE AT BASELINE
AND DURING FOLLOW UP
• APRI (aspartate aminotransferase [AST]-to-platelet ratio index) is recommended as the
preferred non-invasive test (NIT) to assess for the presence of cirrhosis(APRI score >2 in
adults) in resource-limited settings.
• Transient elastography (e.g. FibroScan) or FibroTest may be the preferred NITs in settings
where they are available and cost is not a major constraint
• The FibroTest score is calculated from the results of a six-parameter blood test, combining six serum
markers with the age and gender of the patient: Alpha-2-macroglobulin, Haptoglobin, Apolipoprotein
A1, Gamma-glutamyl transpeptidase (GGT), Total bilirubin, and Alanine transaminase (ALT). ALT is used
in a second assessment called ActiTest that is part of FibroTest.](https://image.slidesharecdn.com/seminarhepbjoy-150820130939-lva1-app6892/85/hepatitis-B-VIRUS-37-320.jpg)