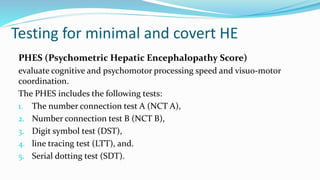
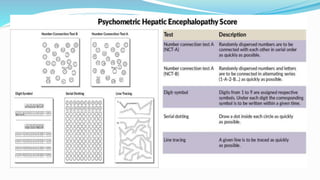
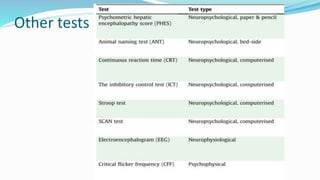
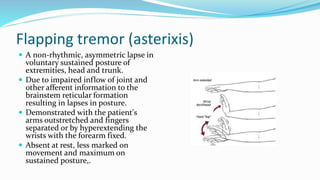
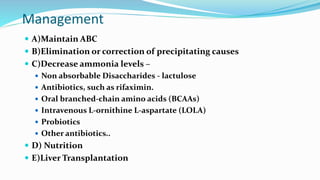
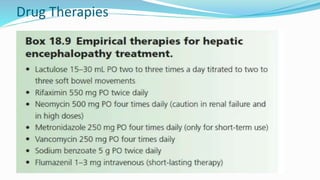
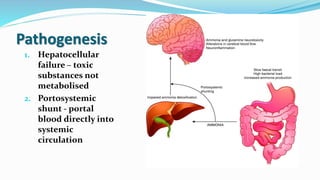
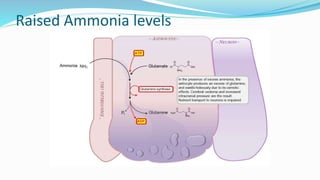
This document provides a comprehensive review of hepatic encephalopathy (HE), including its definition, epidemiology, classifications, clinical features, diagnosis, management, and prognosis. It highlights the role of liver insufficiency and portosystemic shunt in the pathogenesis of HE, noting significant risk factors and the impact of ammonia accumulation in the brain. Additionally, the document outlines diagnostic criteria, treatment options, and preventative measures for managing HE in patients with liver disease.








![Causes
Chronic parenchymal liver disease:
Chronic hepatitis.
Cirrhosis.
Fulminating hepatic failure:
Acute viral hepatitis.
Drugs. E.g Paracetamol overdose
Toxins e.g. Wilson’s Disease.
Surgical Portal-systemic anastomoses
Portacaval shunts, or Transjugular intrahepatic portal-systemic shunting [TIPS]).](https://image.slidesharecdn.com/cmehepaticencephalopathy-210824193147/85/Hepatic-encephalopathy-12-320.jpg)