Embed presentation
Downloaded 76 times


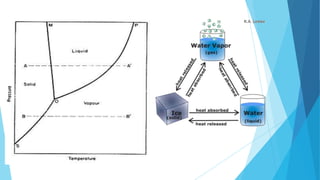

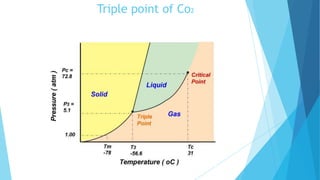
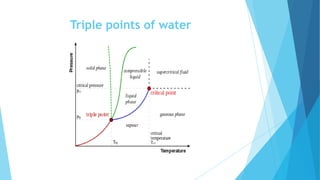

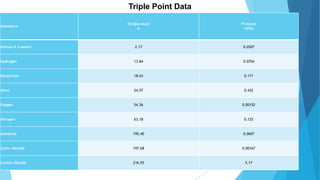

The document discusses the triple point of substances, which is the specific temperature and pressure at which the solid, liquid, and gas phases of a single substance can coexist in equilibrium. It provides the triple point values for water, which is 0.01°C and 611.73 pascals, as well as table listing the triple point temperatures and pressures of various substances including helium, hydrogen, oxygen, nitrogen, ammonia, and carbon dioxide.








