This document provides an overview of heart transplantation, including:
- A brief history of heart transplantation from early experiments in the 1960s to advances like immunosuppressant drugs and ventricular assist devices.
- Stages of heart failure from risk factors to end-stage disease requiring specialized interventions.
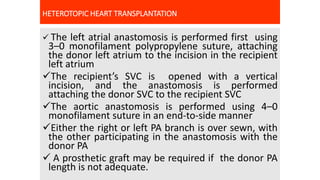
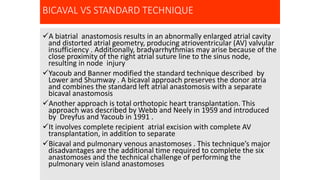
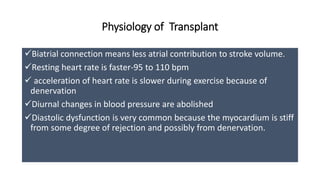
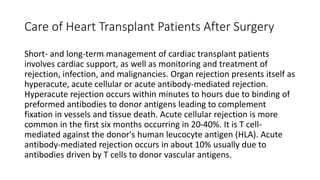
- Key details on the surgical procedure for heart transplantation and long-term patient care, including monitoring and treating rejection, infection, and other complications.
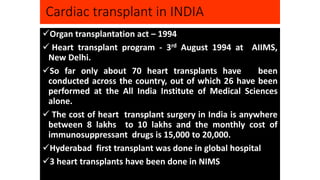
- India has conducted around 70 heart transplants since 1994, though availability remains limited due to costs and immunosuppressant drug expenses.

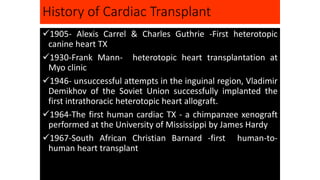
![History Brief
Heart transplantation became a reality in the late 1960s after nearly a half of a century of
research in surgical techniques, pathophysiology, and immunology by Drs. Richard Lower
and Norman Shumway. Dr. Shumway is regarded as the father of heart transplantation. Dr.
Christiaan Bernard, a student of Lower, performed the first heart transplantation in the
laboratory on December 3, 1967. Dr. Adrian Kantrowitz performed the first pediatric
transplant on Dec 6, 1967 in New York. He also laid the foundation for formulating the
brain death criteria, which later came out of the Ad Hoc Committee of the Harvard Medical
School. The early results were quite dismal, and many centers were forced to close their
programs. In the late 1970s, survival improved to six years. Major advances in survival
should be credited to Dr. Margaret Billingham for standardization of pathological diagnosis
of rejection and later development of anti-rejection medicines. In 1983, the U.S. Food and
Drug Administration (FDA) approved cyclosporin after its discovery by the Sandoz team. In
October 1994, ventricular assist devices (VADs) were approved for supporting patients to
transplant (bridge to transplantation [BTT]). In October 2014, Dr. Kumud Dhital of Australia
transplanted a heart that was resuscitated after cardiac death of the donor, opening a new
door to increasing the number of organs.](https://image.slidesharecdn.com/hearttransplantation-141123111018-conversion-gate02/85/Heart-transplantation-2-320.jpg)
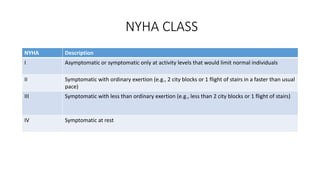
![Clinicopathological Stages
Stages Description
A Patients at high risk (e.g., with hypertension, atherosclerotic disease, diabetes mellitus,
metabolic syndrome, cytotoxin, family history) for HF but without structural heart
disease or symptoms of HF
B Patients with structural heart disease (e.g., left ventricular [LV] dysfunction) but
without symptoms of HF
C Patients with structural heart disease with prior or current symptoms of HF
D Patients with refractory HF requiring specialized interventions](https://image.slidesharecdn.com/hearttransplantation-141123111018-conversion-gate02/85/Heart-transplantation-3-320.jpg)



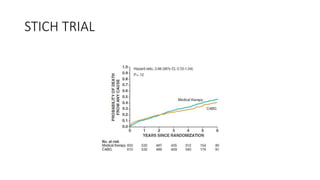


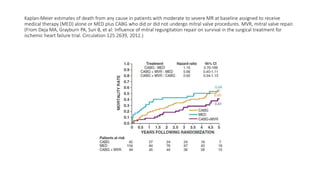
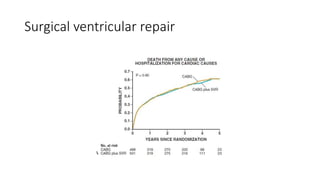





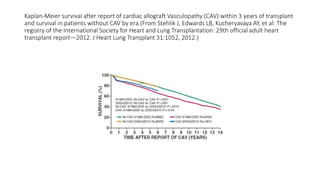

![Future of stage-D heart failure
The most widely used surgical procedure for heart failure is
CABG[STICH]
Drop in operative/post op mortality with surgery
VAD
TAVR
Of course heart transplantation in developing heart](https://image.slidesharecdn.com/hearttransplantation-141123111018-conversion-gate02/85/Heart-transplantation-29-320.jpg)


