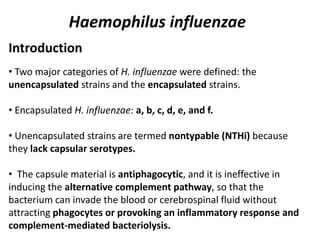
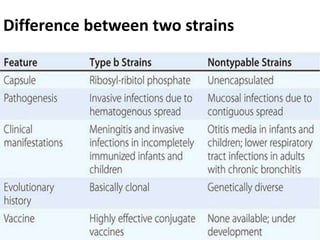
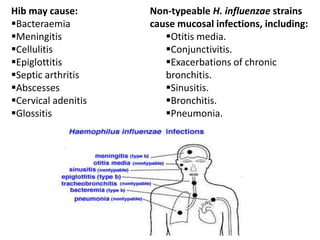
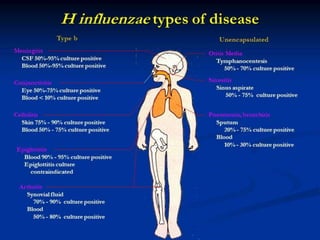
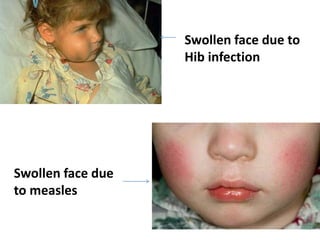
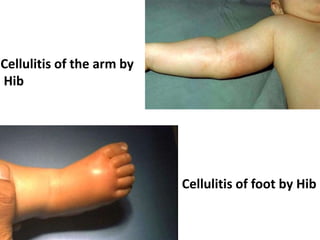
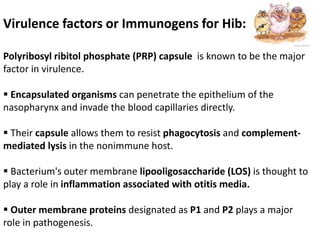
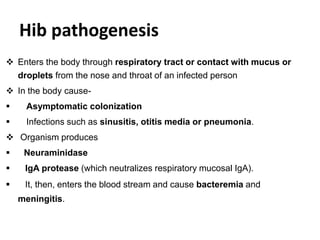
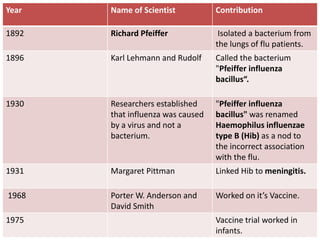
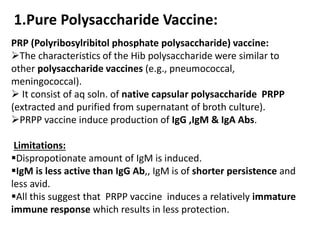
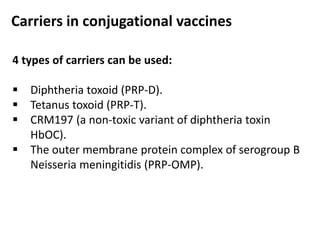
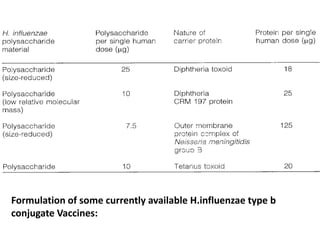
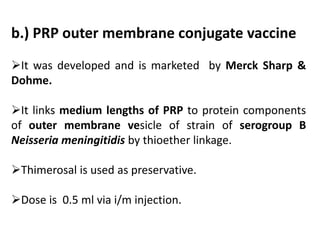
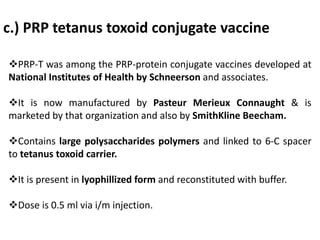
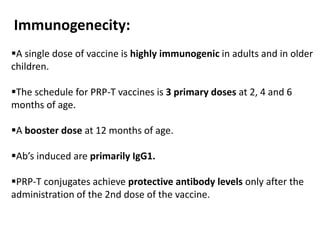
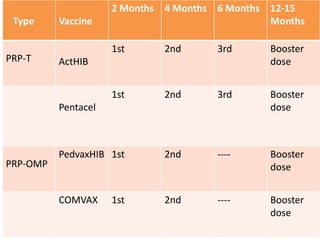
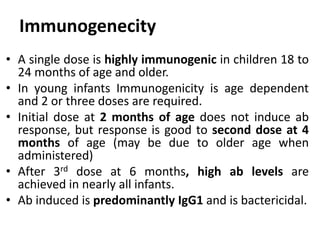
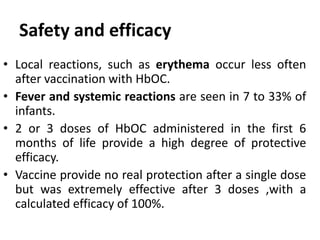
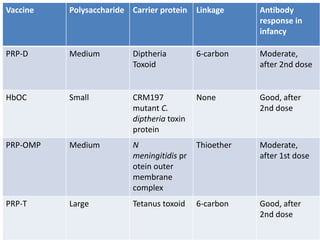
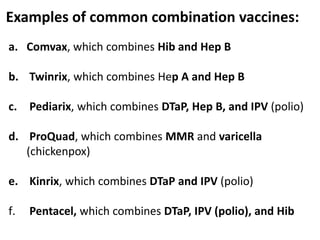
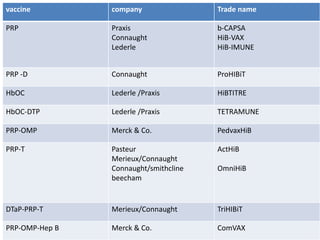
Haemophilus influenzae is a bacterium that exists in two forms - encapsulated strains including types a-f, and unencapsulated nontypable strains. Encapsulated strains like Hib can cause serious invasive diseases while nontypable strains typically cause mucosal infections. Prior to vaccination, Hib was a leading cause of bacterial meningitis and death in young children. Effective conjugate vaccines were developed that link the Hib polysaccharide to carrier proteins, stimulating immune memory and higher antibody levels compared to unconjugated vaccines. Common Hib vaccines include PRP-D, PRP-T, PRP-OMP, and HbOC combinations, which provide protection through 3-4 doses in