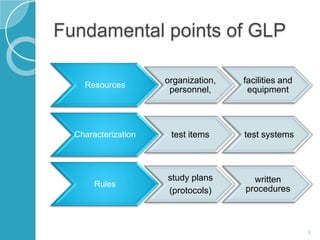
GLP regulations were established in the 1970s in response to issues in pharmaceutical R&D. GLP provides a quality system for non-clinical studies by requiring organization, facilities, personnel, test characterization, study plans, and record-keeping. Key aspects of GLP include defining organizational structure and staff responsibilities, ensuring adequate facilities and equipment, documenting test materials and systems, having written protocols and procedures, recording raw data, archiving records, and independent quality assurance oversight of studies. GLP applies primarily to safety studies for products regulated by drug authorities.