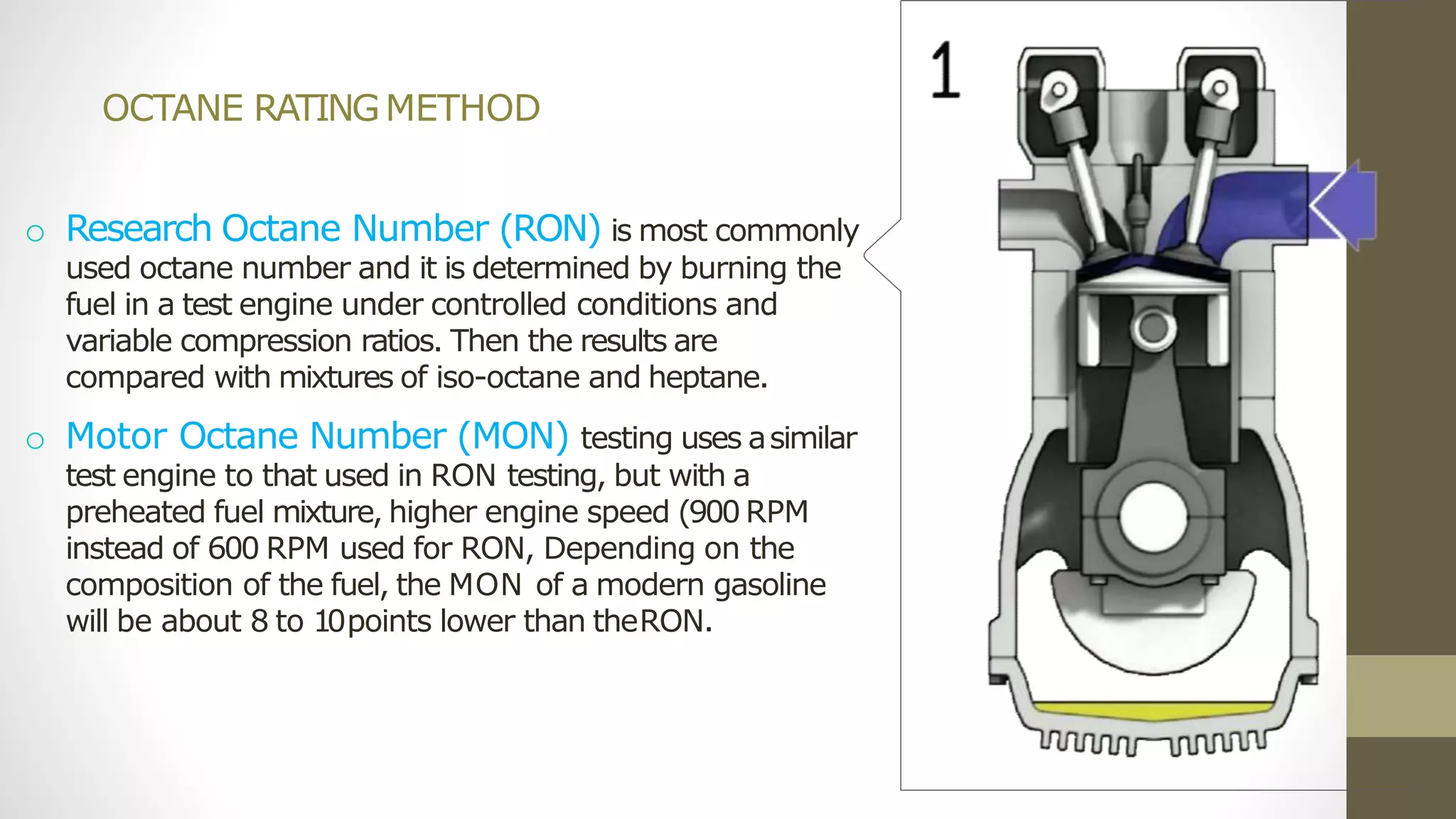
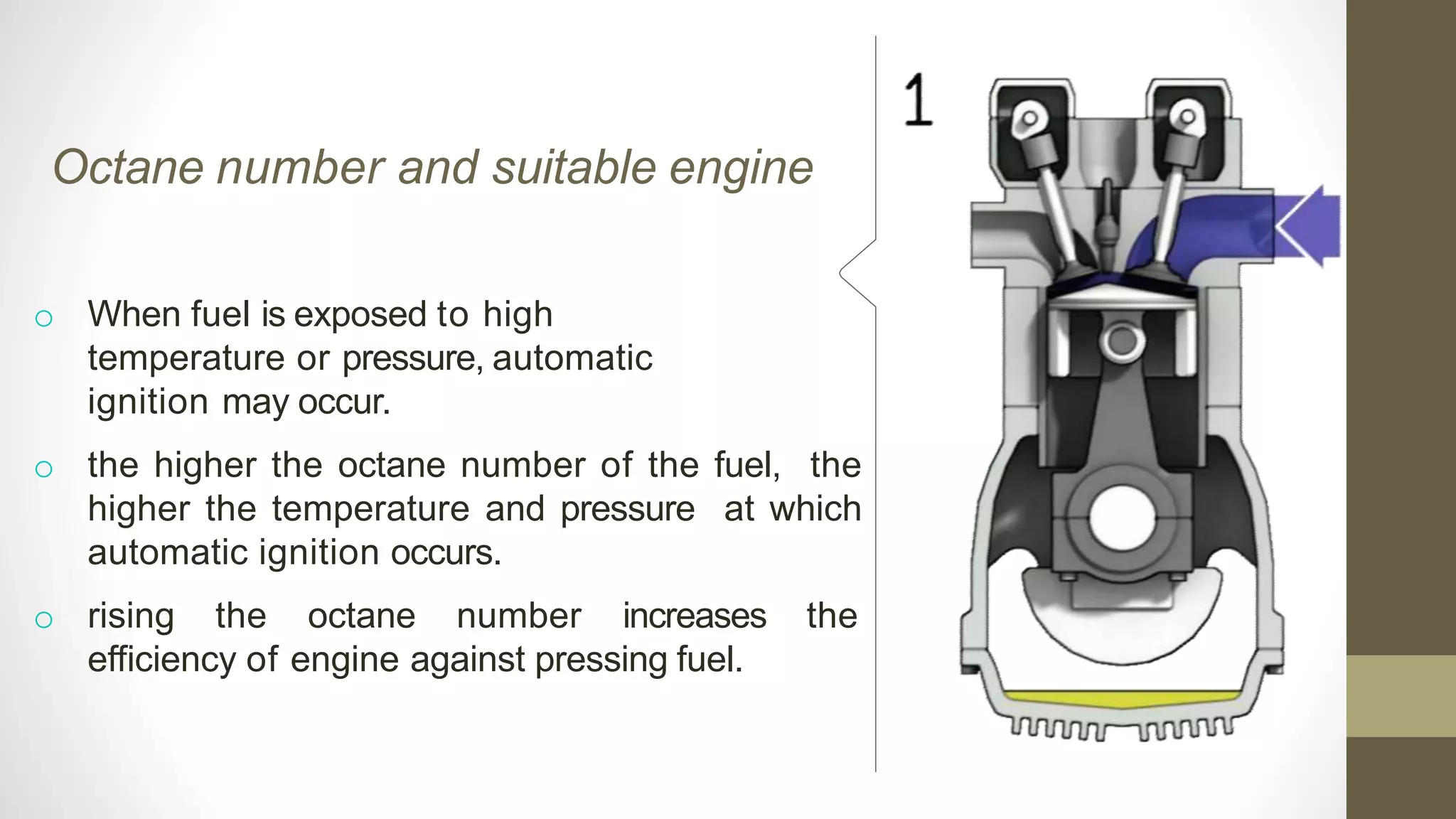
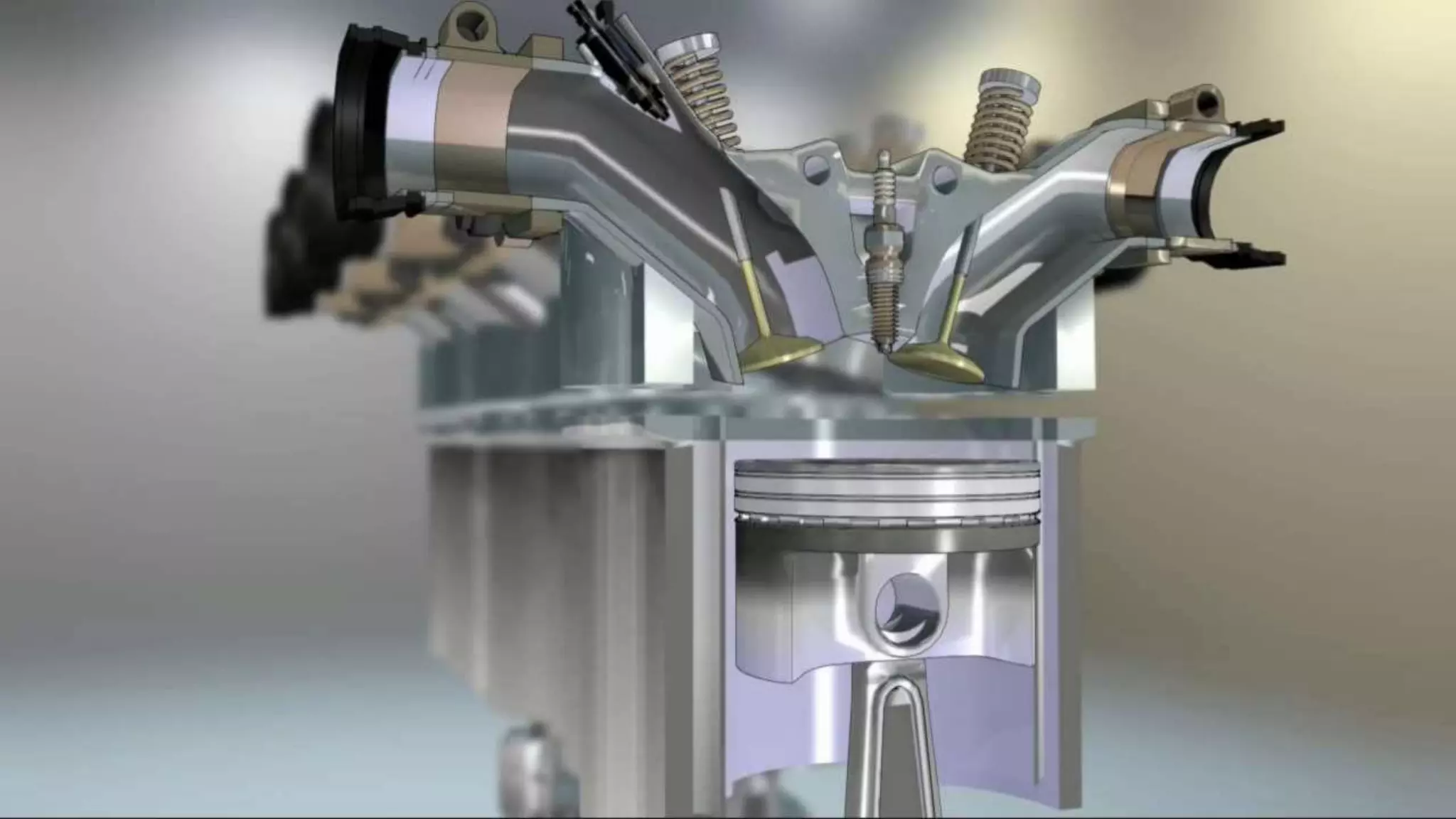
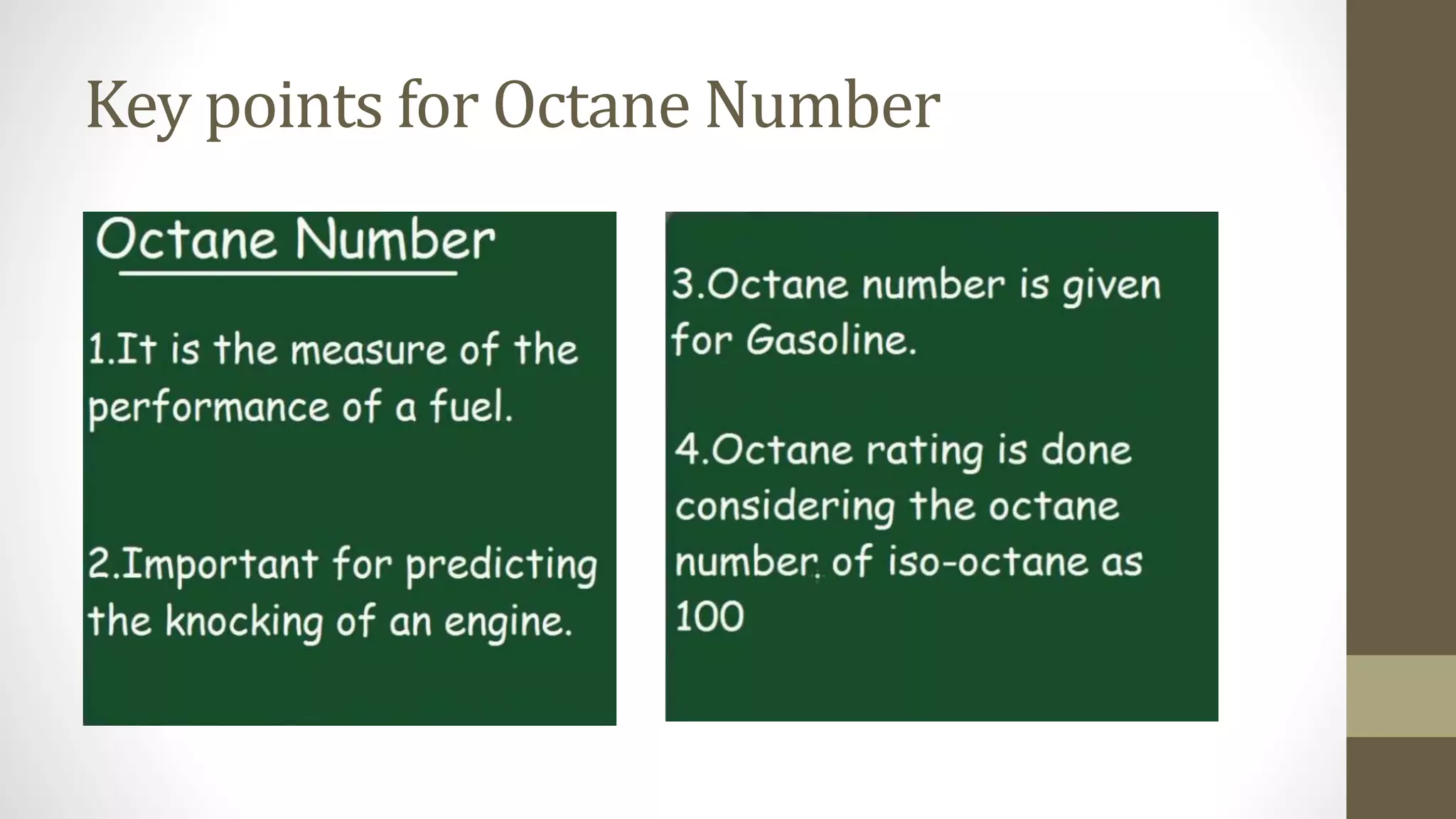
The document discusses various aspects of engine knock, its origins, and factors influencing it, including the importance of octane and cetane numbers in fuels. It highlights knock measurement techniques, damage caused by knock, and strategies for controlling it in SI and CI engines. Additionally, the document touches on the classification and characteristics of fuels, methods to improve their octane ratings, and mentions octane boosters and Shell's V-Power fuel technology.






![Knock measurement [sensors]
• Optical probes and Ionization detectors can
be used
• Piezoelectric pressure transducer is used
mostly
• Generate a voltage when vibration is applied
to them utilizing the piezoelectric effect and it
is proportional to the acceleration
• Due to the vibration, a counter weight inside
the sensor is applying pressure on the piezo
element, this pressure creates an electric
charge in the piezo element which is the
output signal of the sensor.](https://image.slidesharecdn.com/knockingcetaneandoctanenumer-210504134057/75/Knocking-cetane-and-octane-numer-9-2048.jpg)