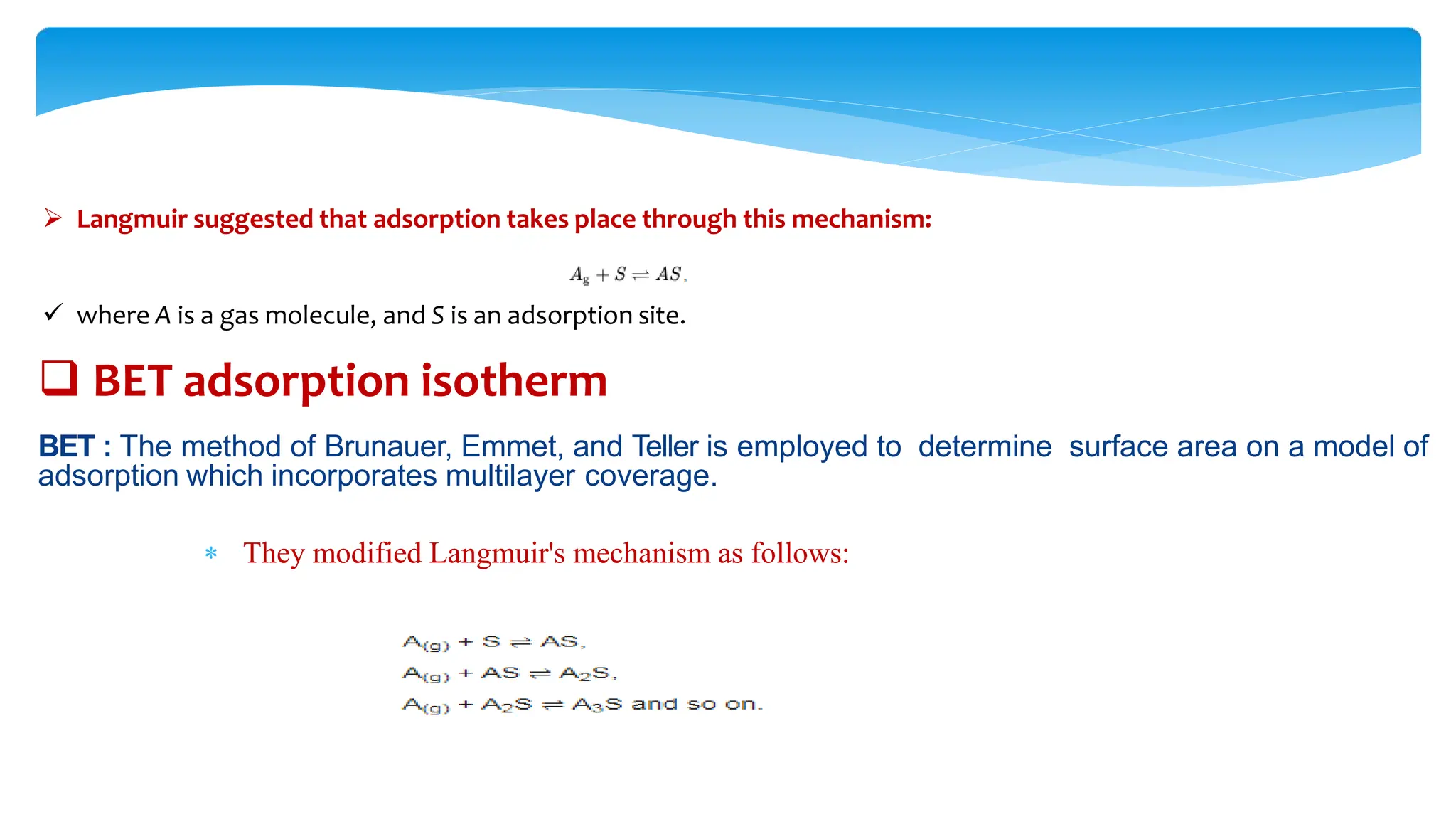
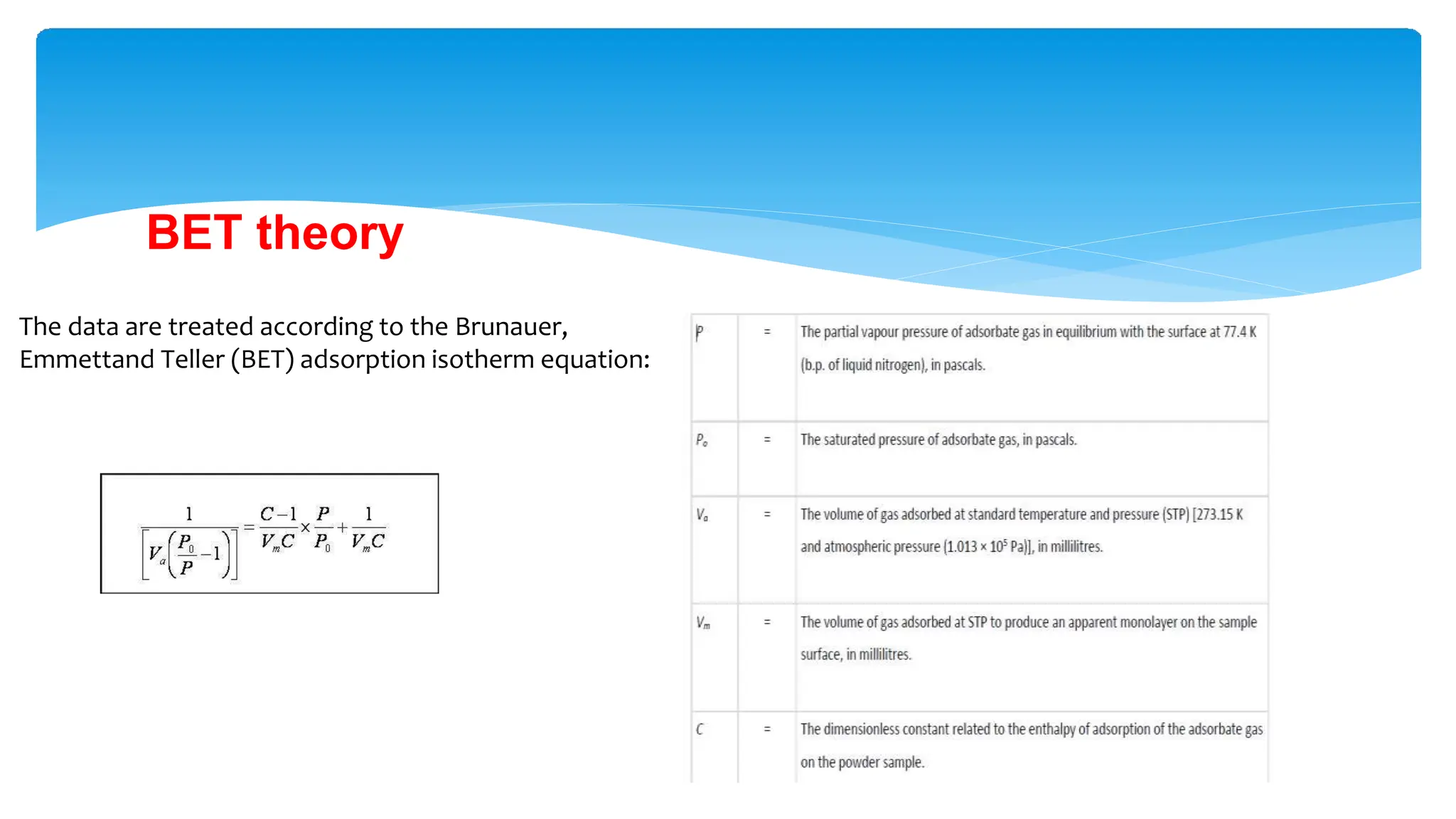
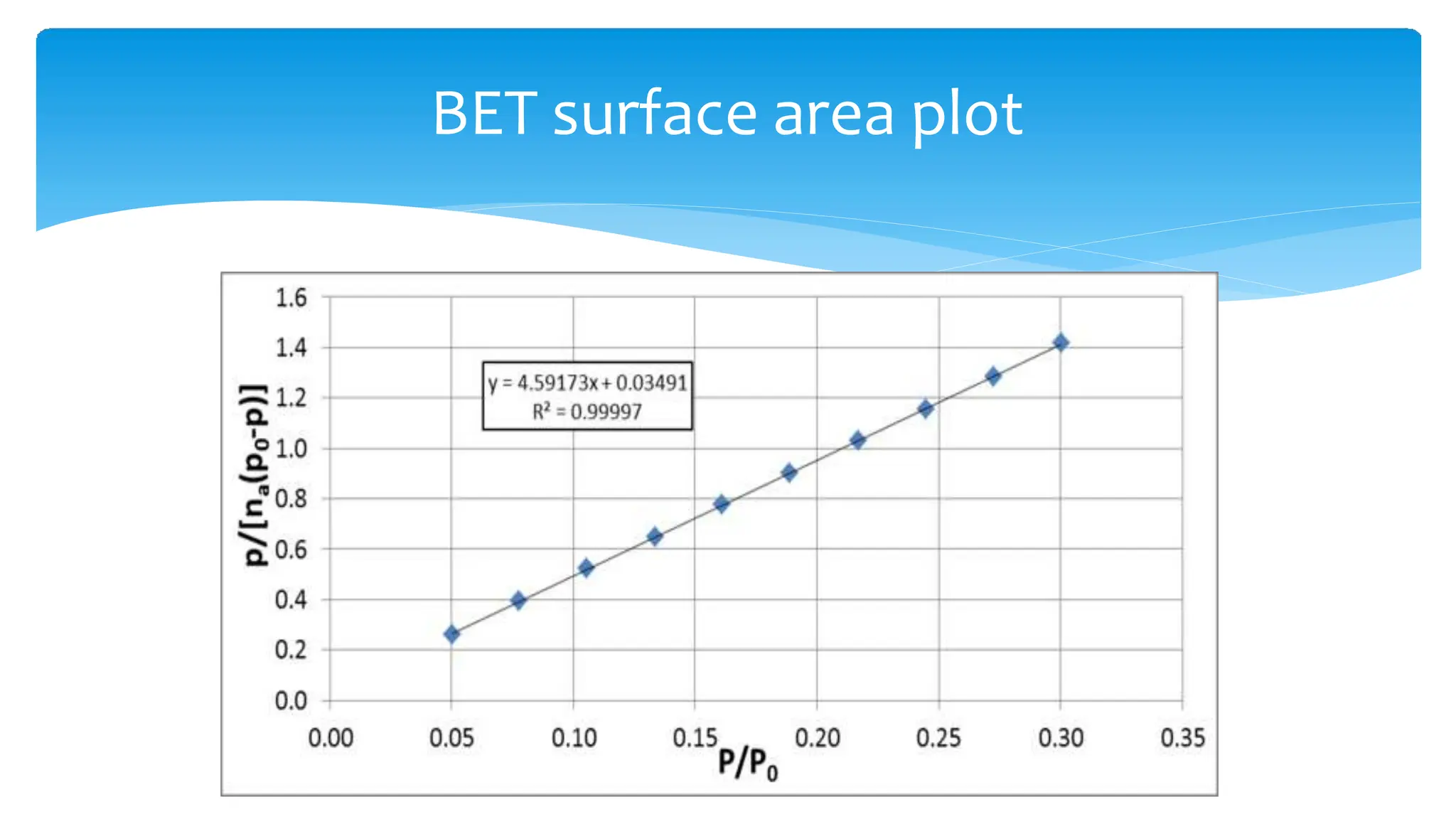
The document discusses surface area analysis using the Brunauer-Emmett-Teller (BET) method, which is used to determine surface areas and pore sizes through gas adsorption. It elaborates on different adsorption isotherms, including Langmuir and BET theories, and the empirical models they provide for understanding adsorption behavior on solid surfaces. Additionally, it describes the methodologies for conducting experiments to measure surface area and the classification of pore sizes.