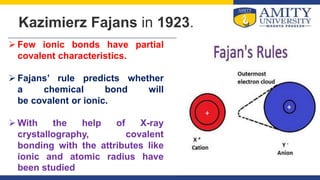
Fajan's rules provide a framework for predicting the ionic or covalent nature of chemical bonds based on three main factors:
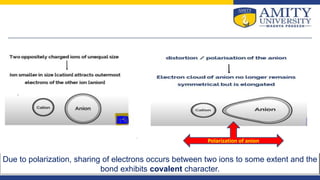
1. Smaller cation size and larger anion size leads to greater covalent character, as the cation can more strongly polarize the anion.
2. Higher cation charge leads to greater covalent character, as a more charged cation can more strongly polarize the anion.
3. For cations of similar size and charge, those with electronic configurations like transition metals that are less able to shield charge will more strongly polarize the anion and have greater covalent character.
The rules are applied in examples where LiCl is predicted to have the most co