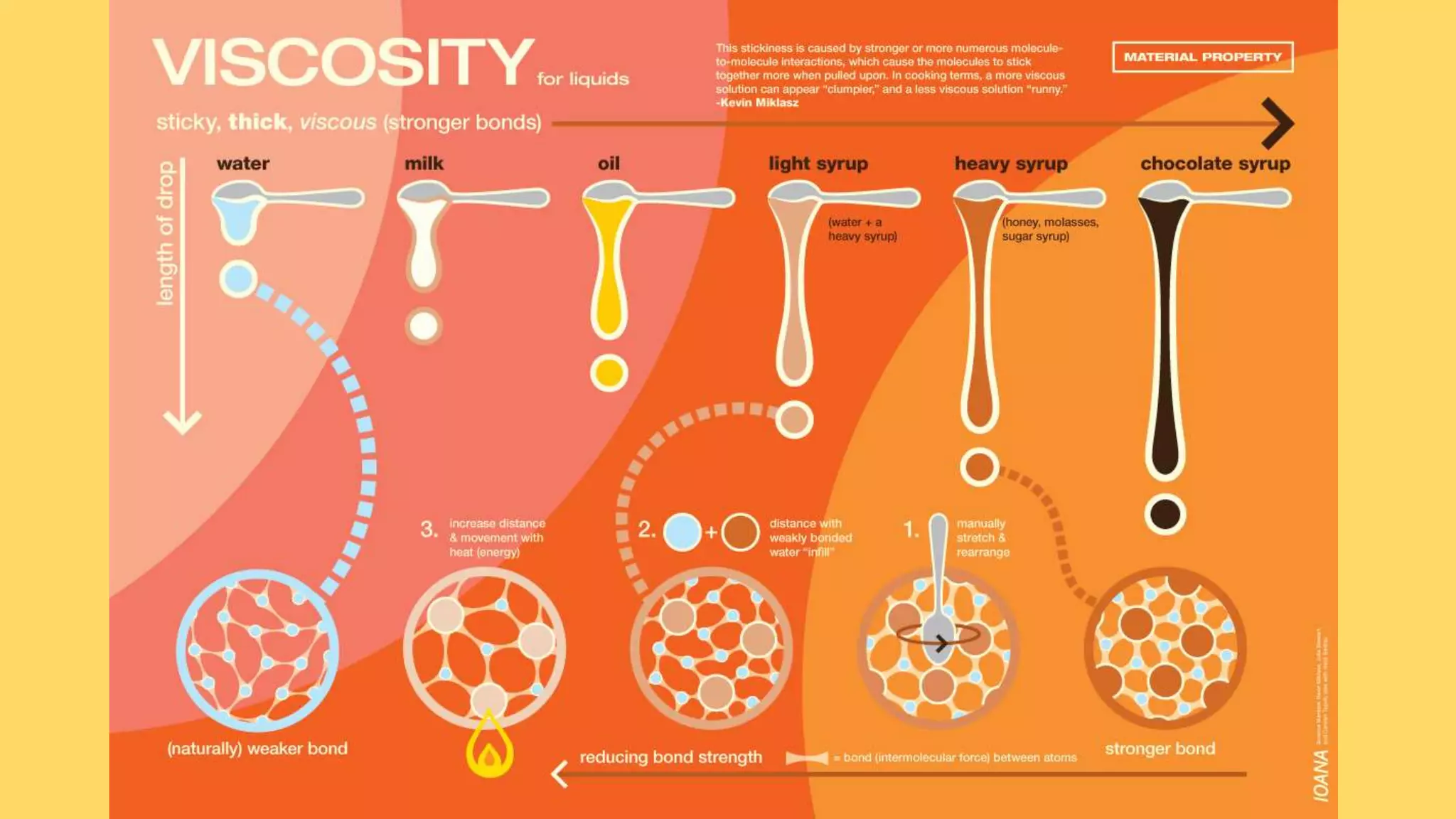
Viscosity is a measure of a liquid's resistance to flow. The viscosity of a solution relates to how strongly the solute molecules interact with each other and resist an applied shear stress. Viscosity depends on factors like the size, shape, flexibility, and hydration of solute molecules. High molecular weight polymers and proteins can greatly increase viscosity even at low concentrations. Most protein solutions do not behave according to Newtonian fluid dynamics and instead display shear thinning or pseudoplastic behavior where viscosity decreases with increasing shear rate. The viscosity of protein solutions is influenced by molecular interactions between proteins as well as protein-solvent interactions and hydrodynamic volume.