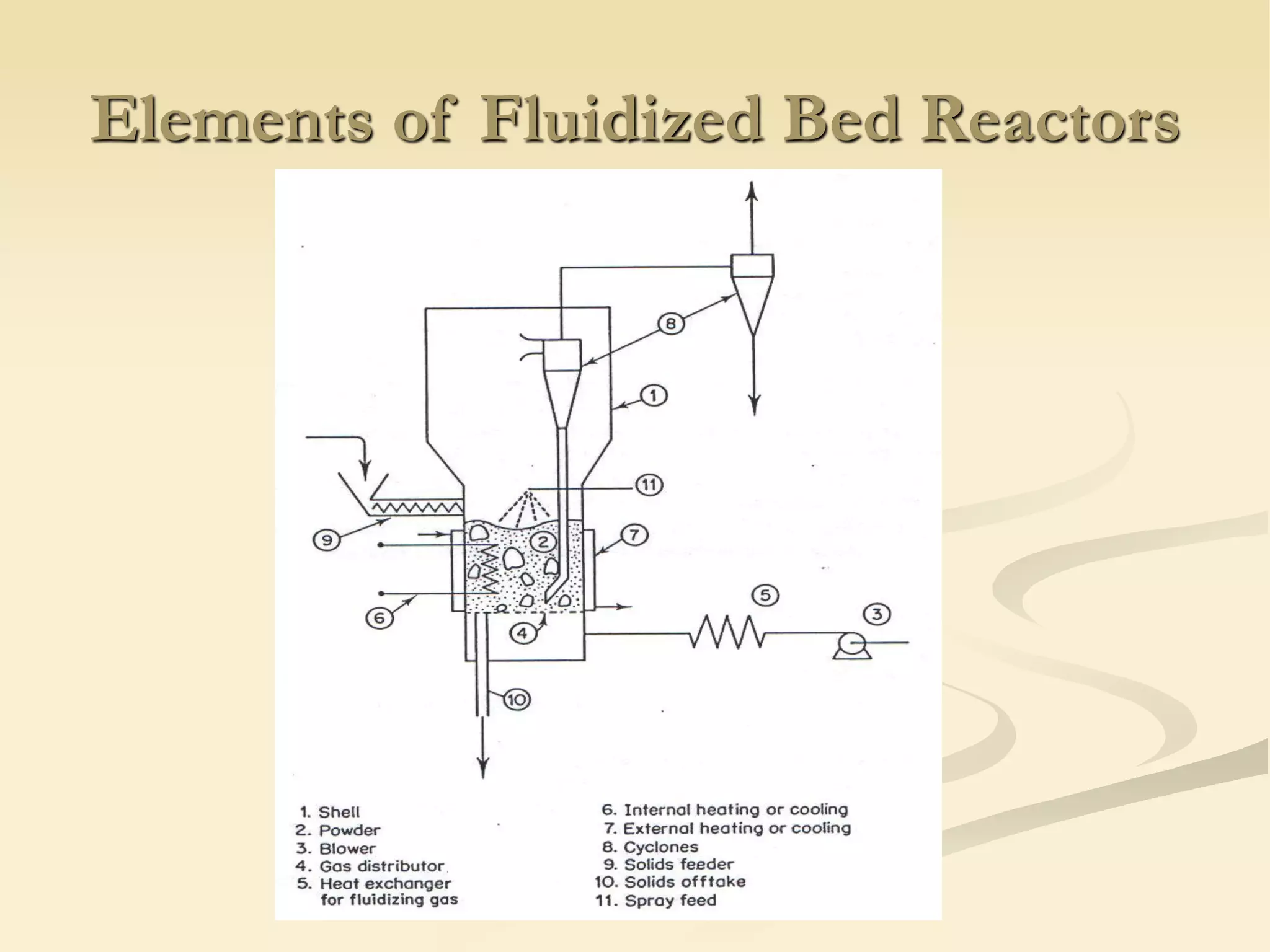
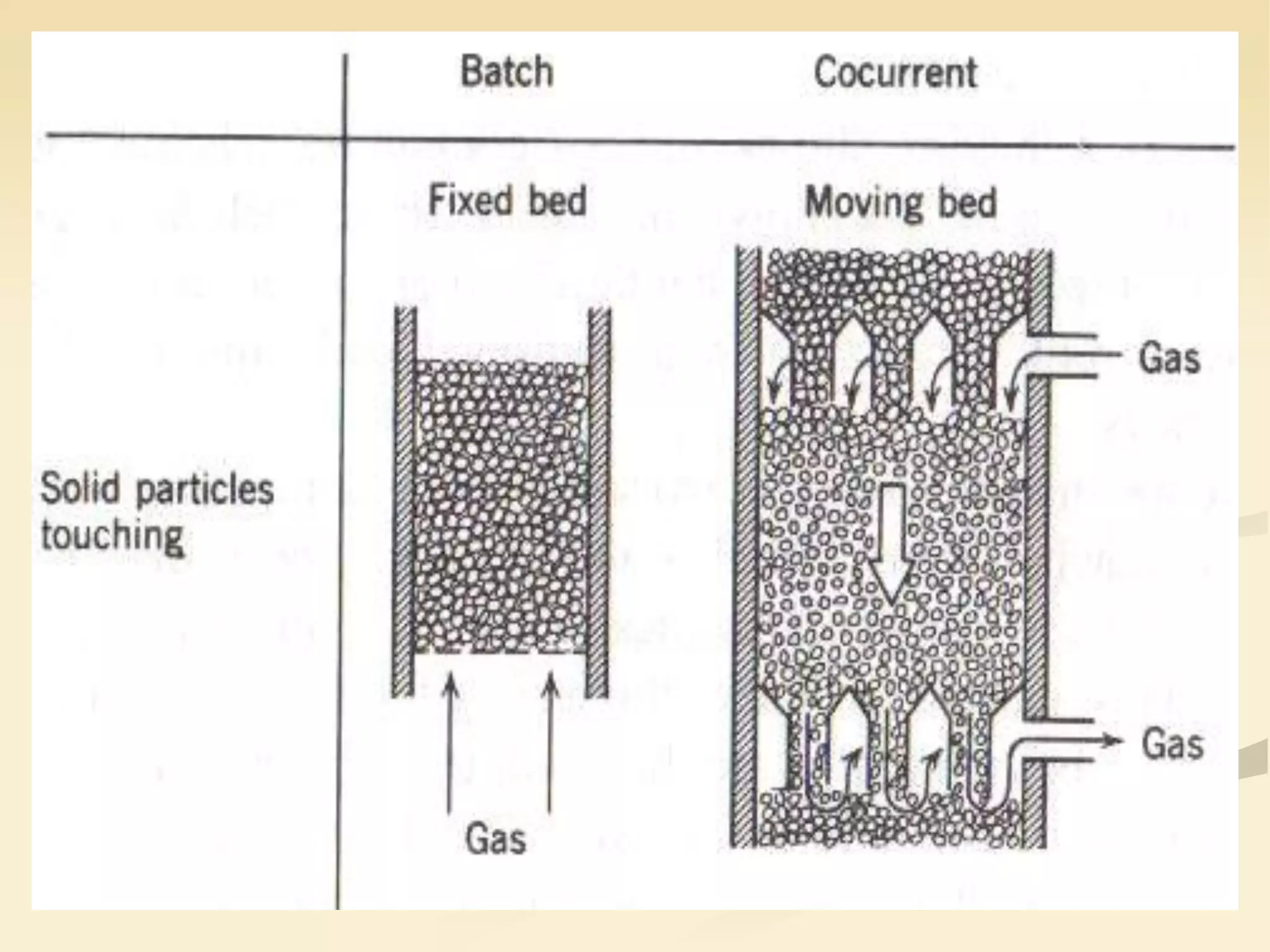
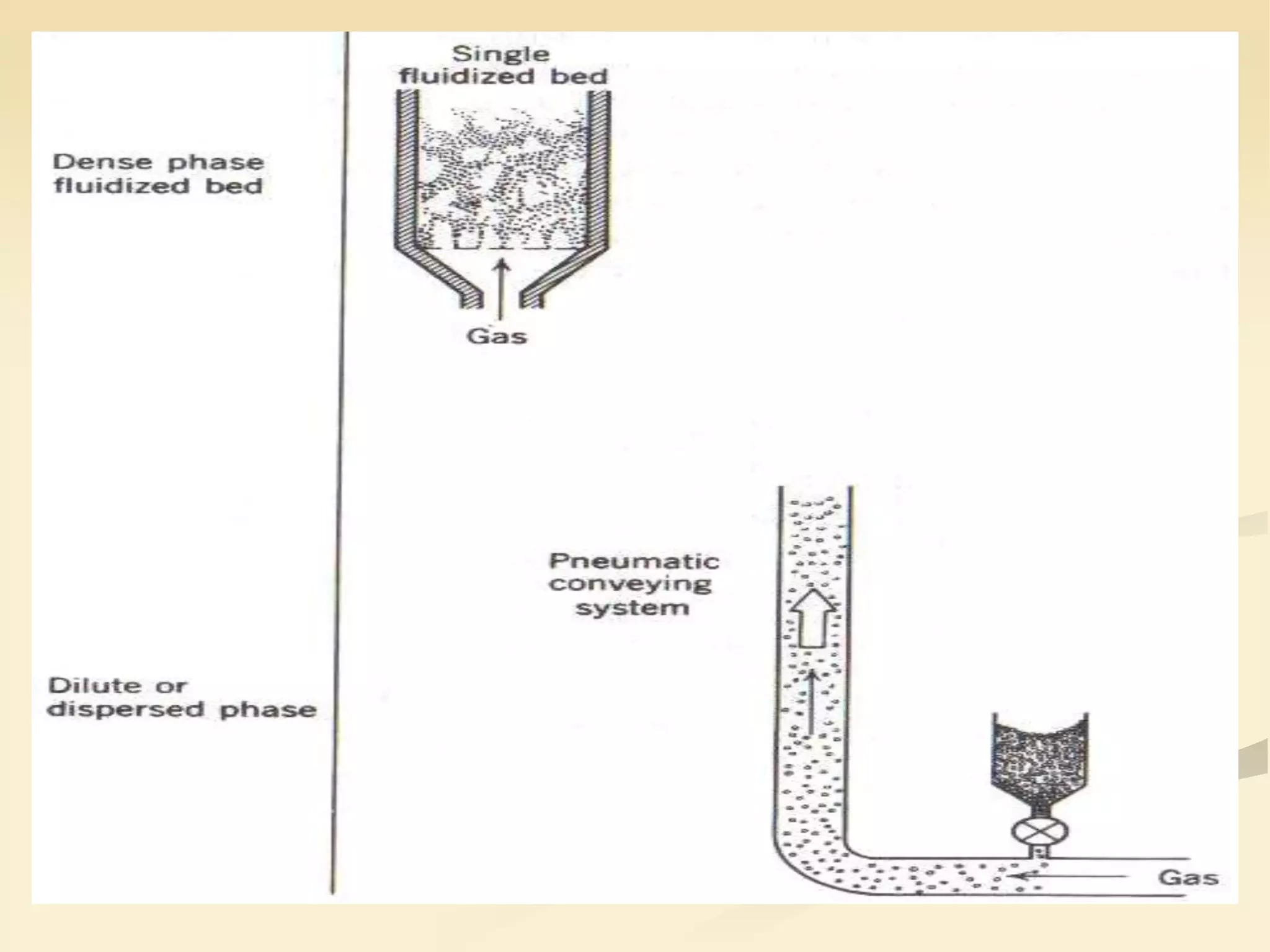
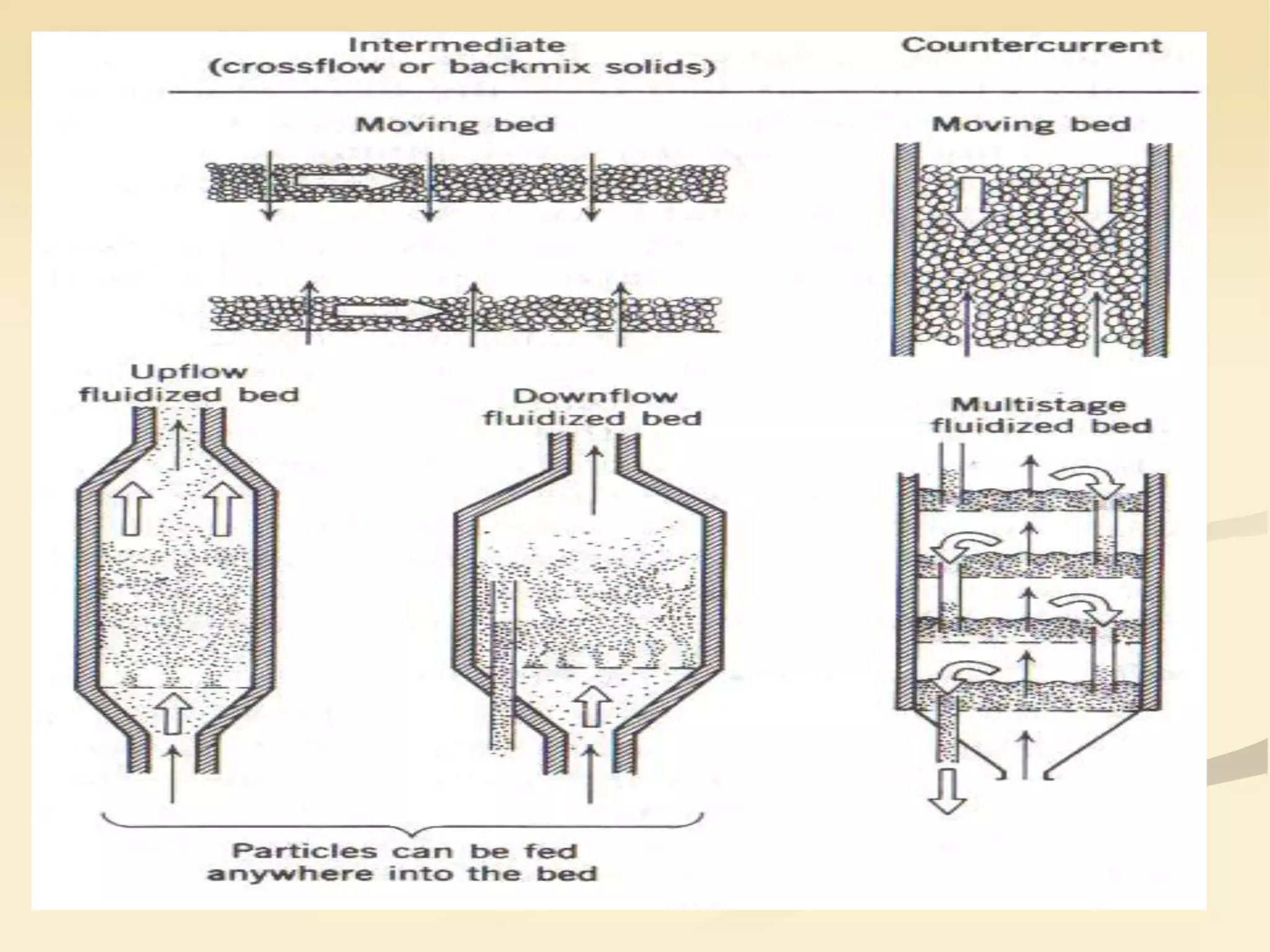
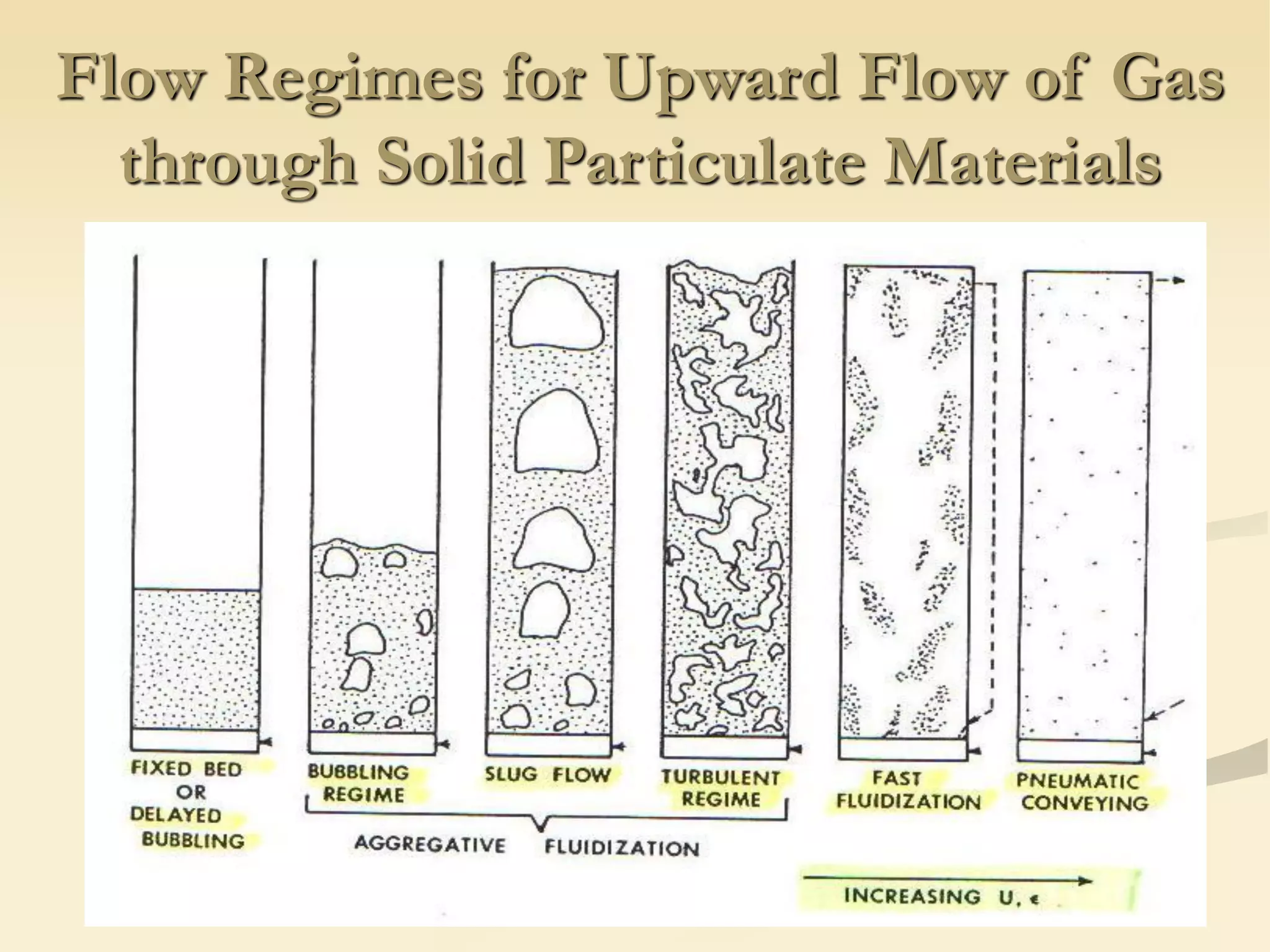
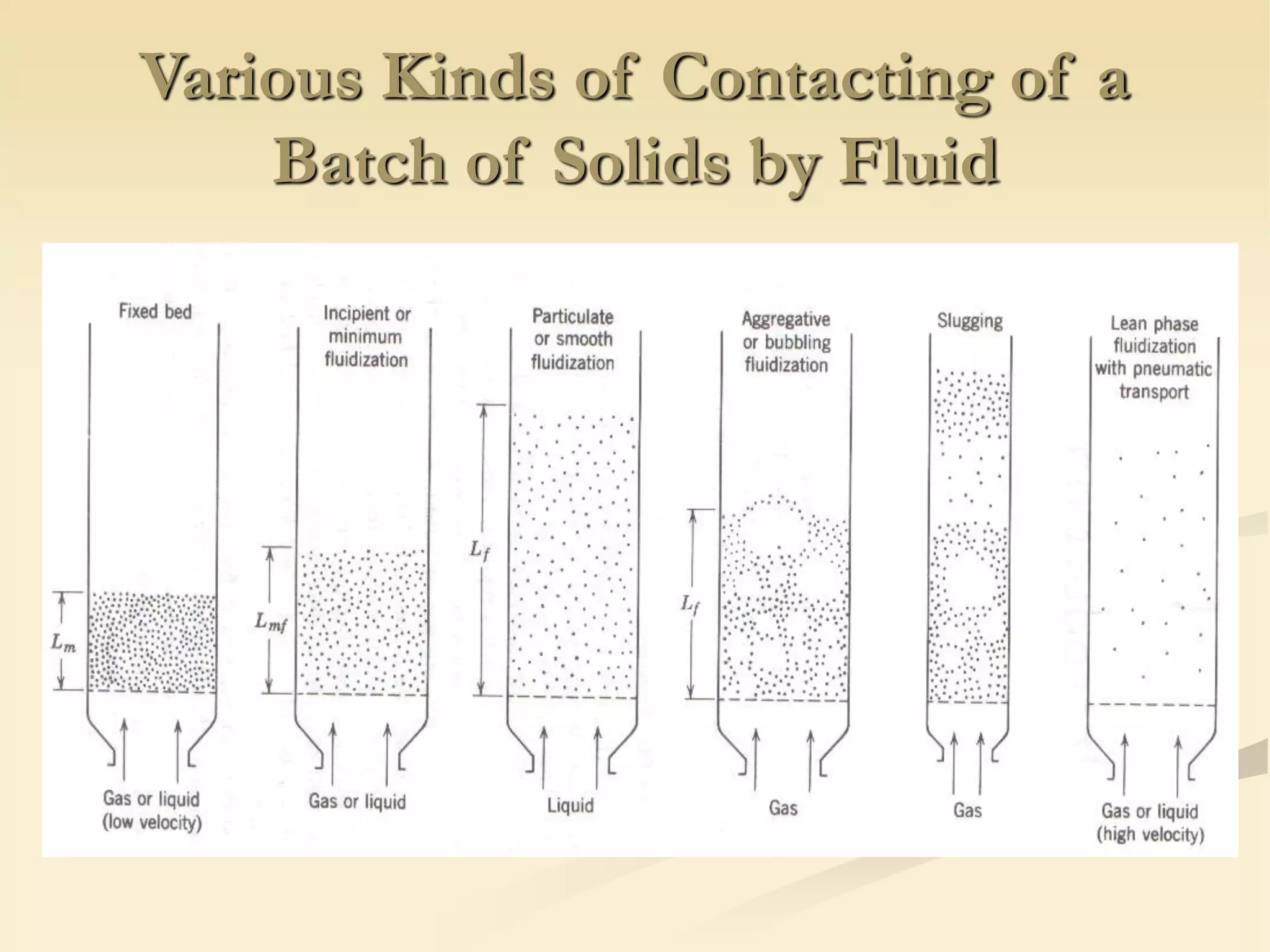
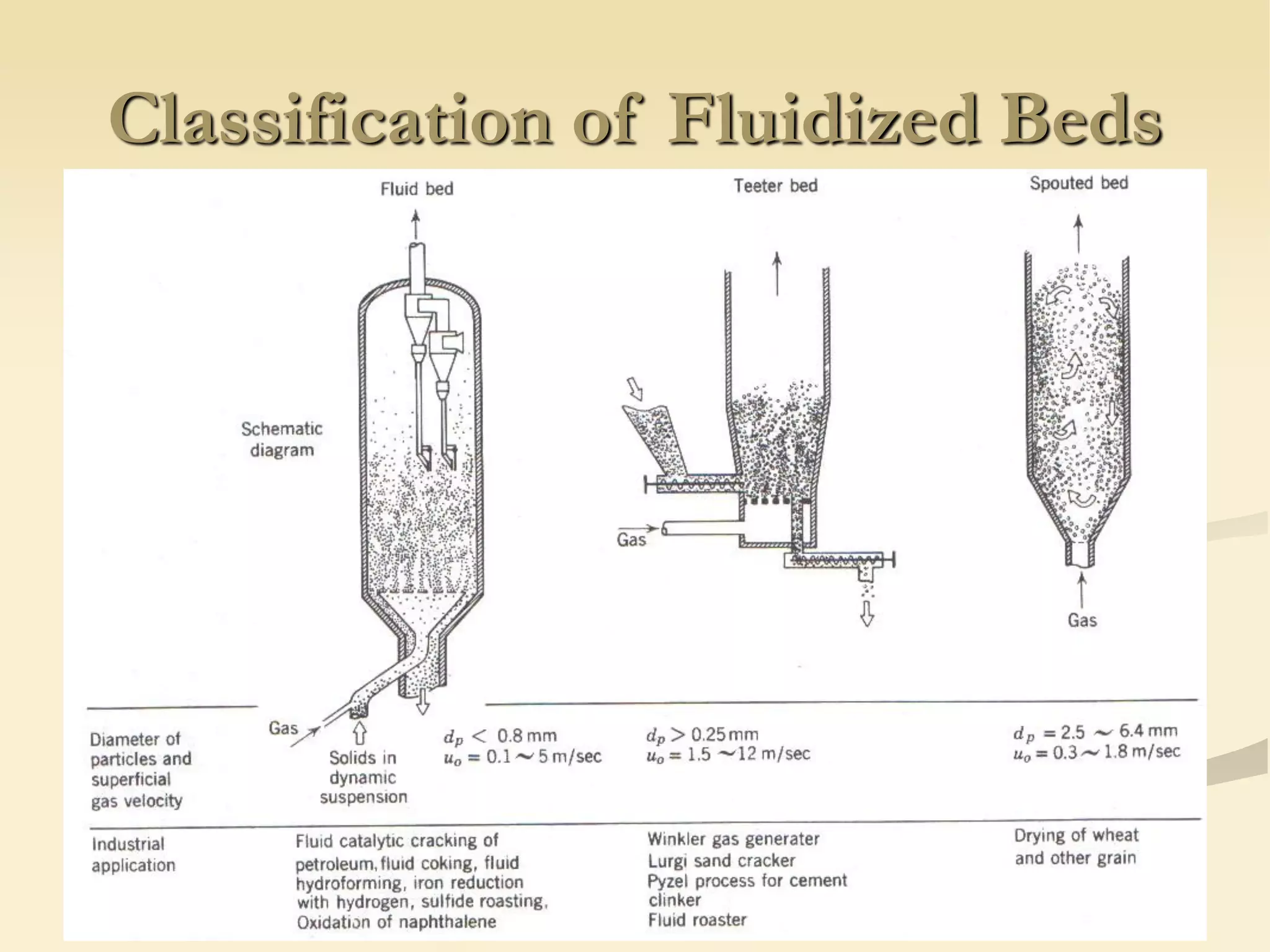
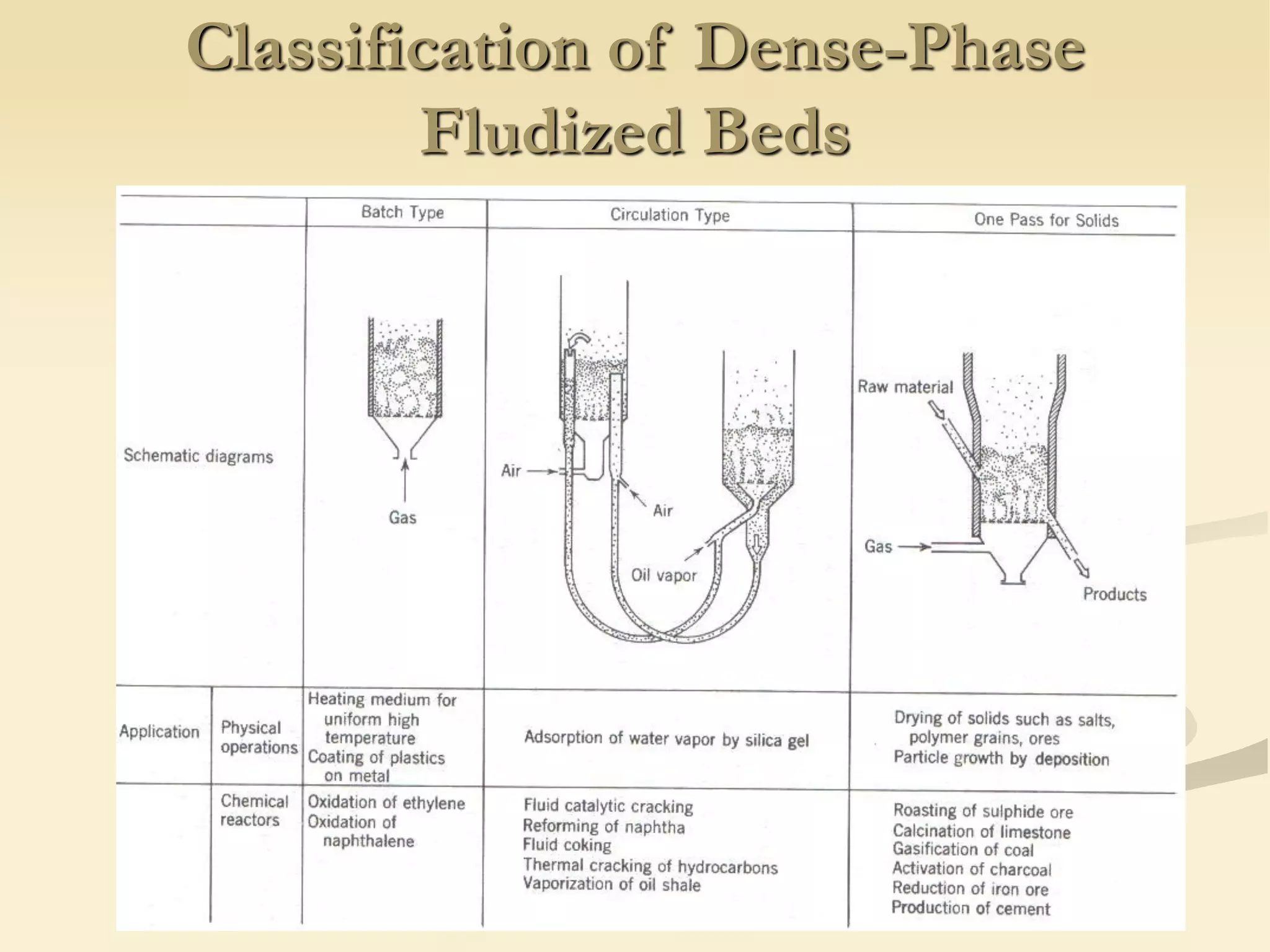
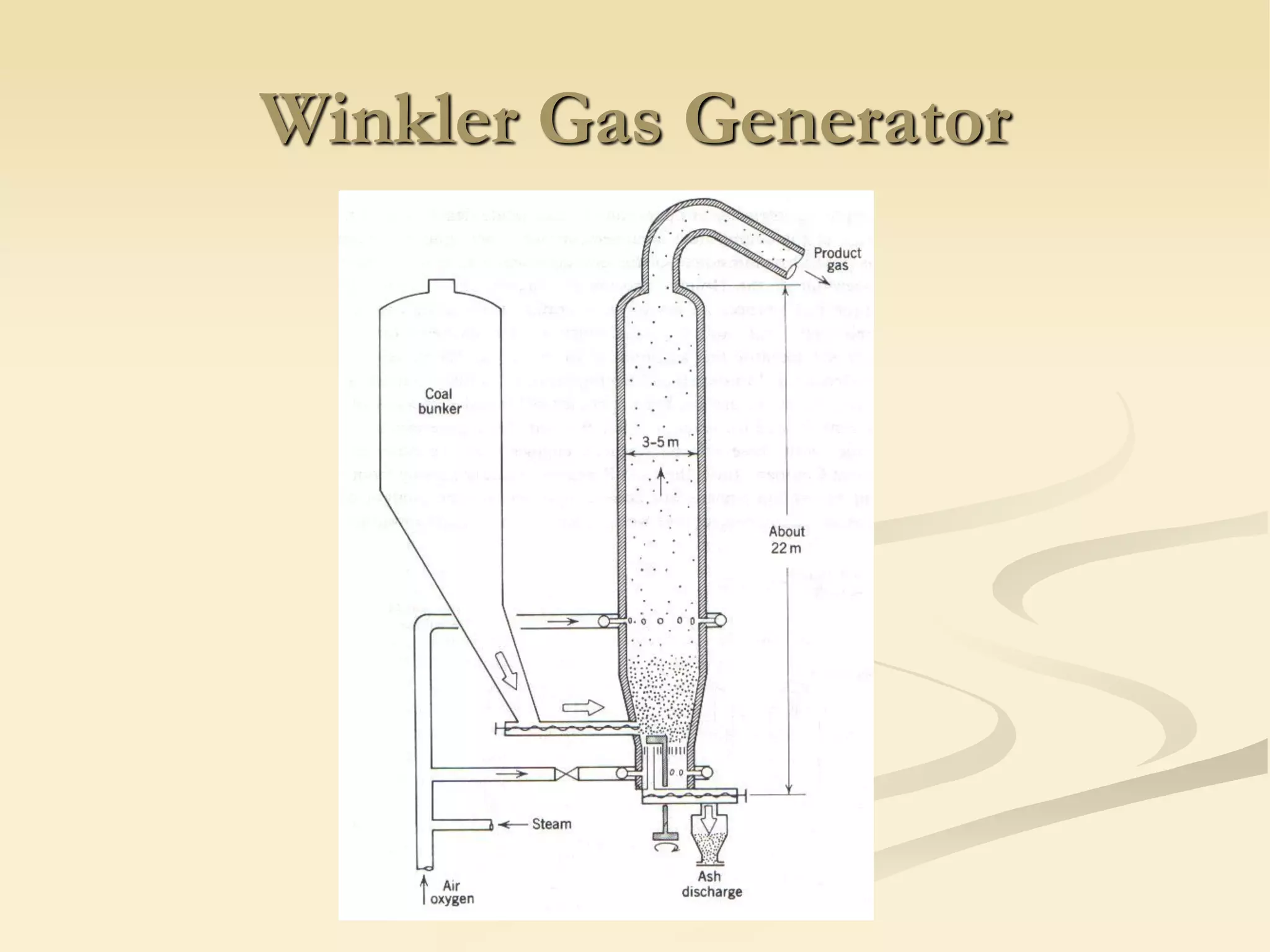
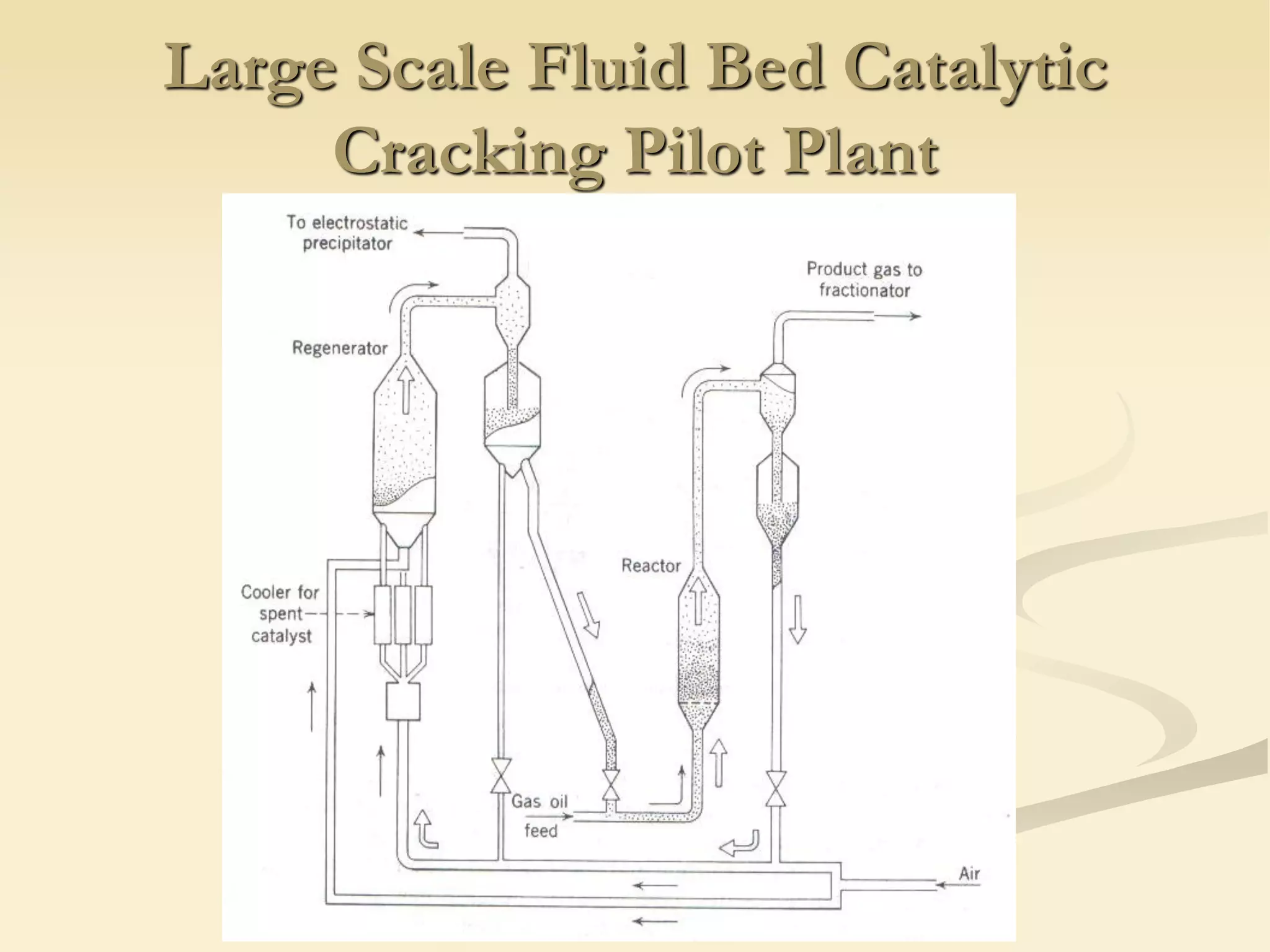
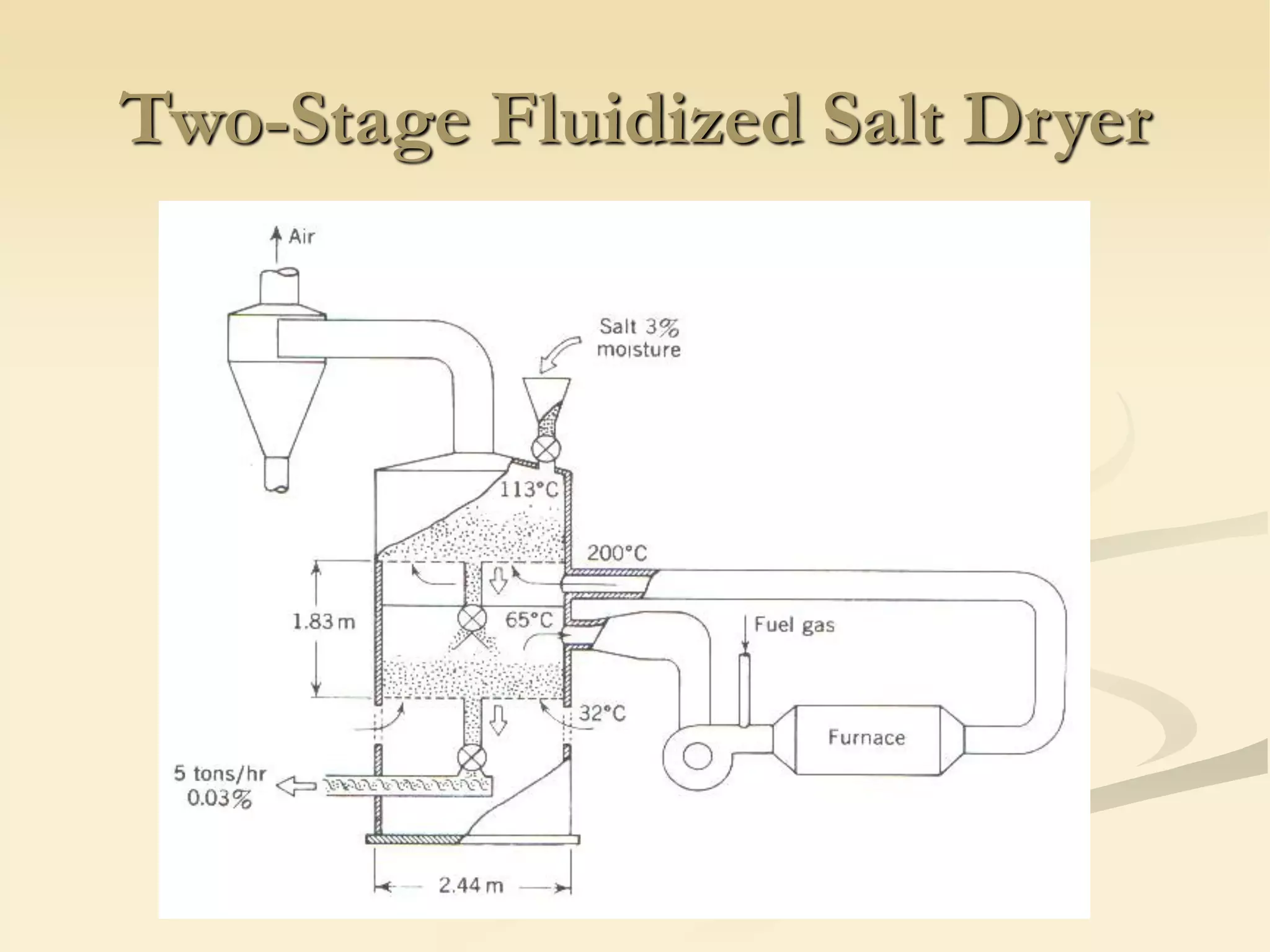
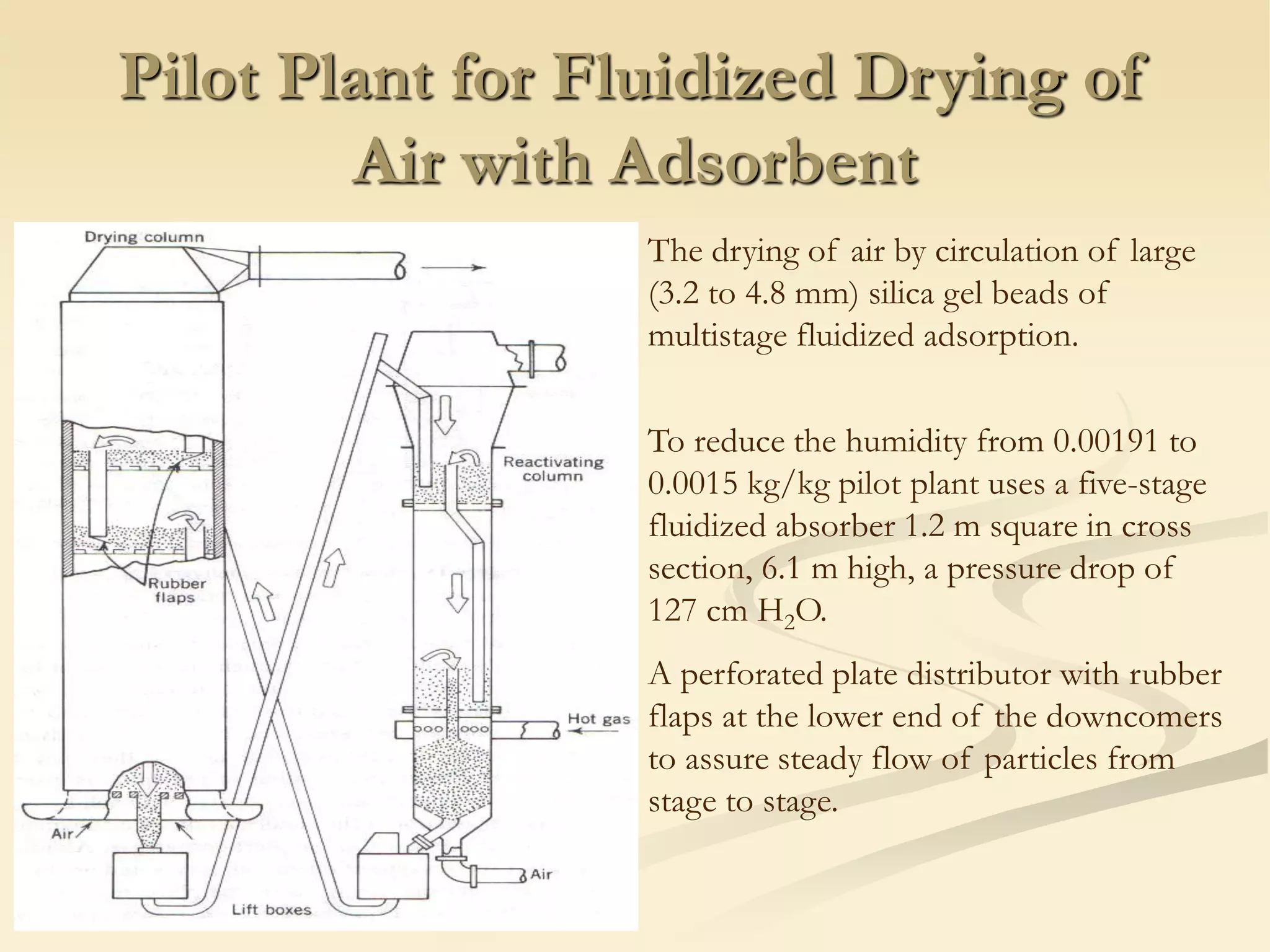
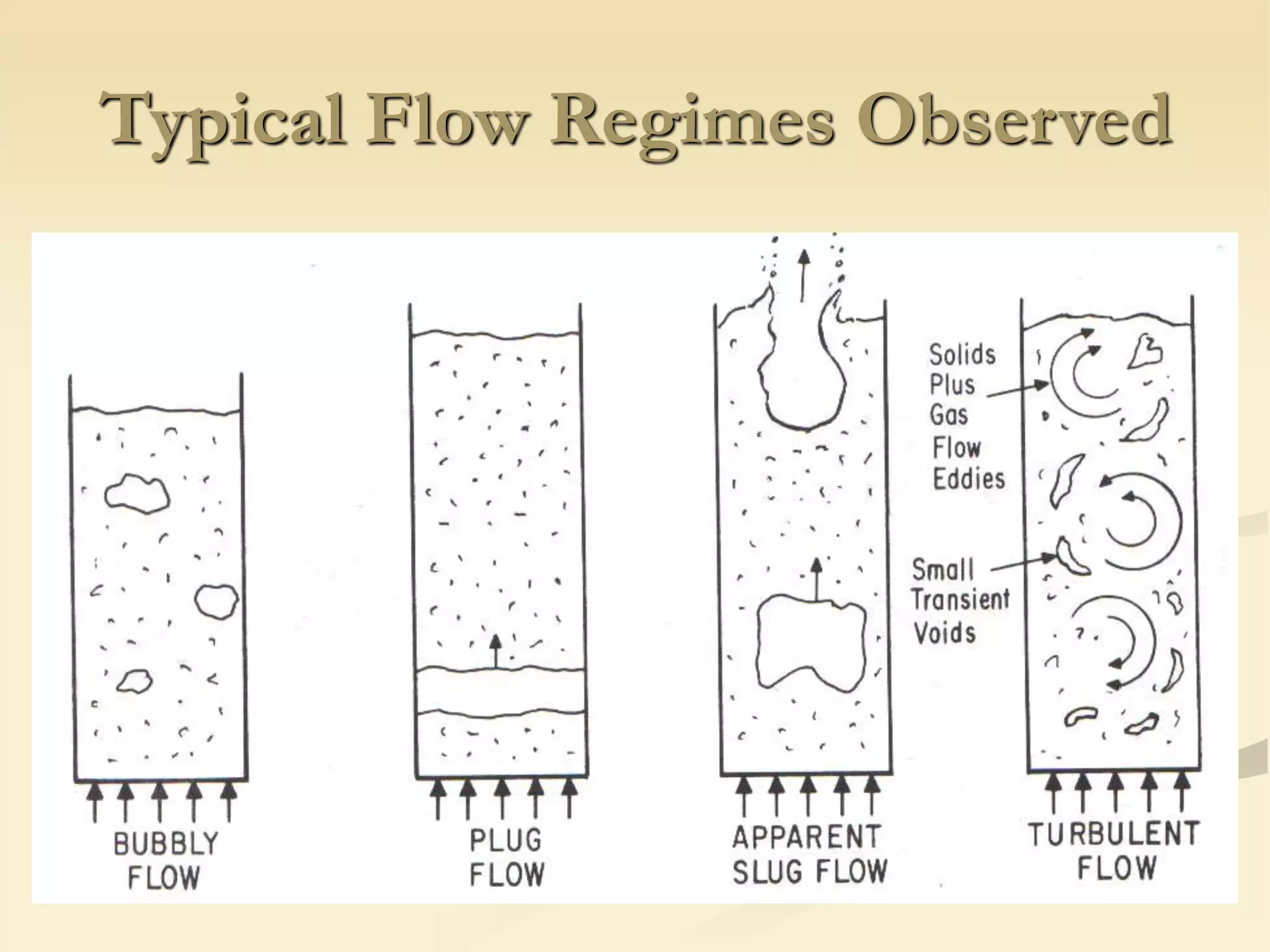
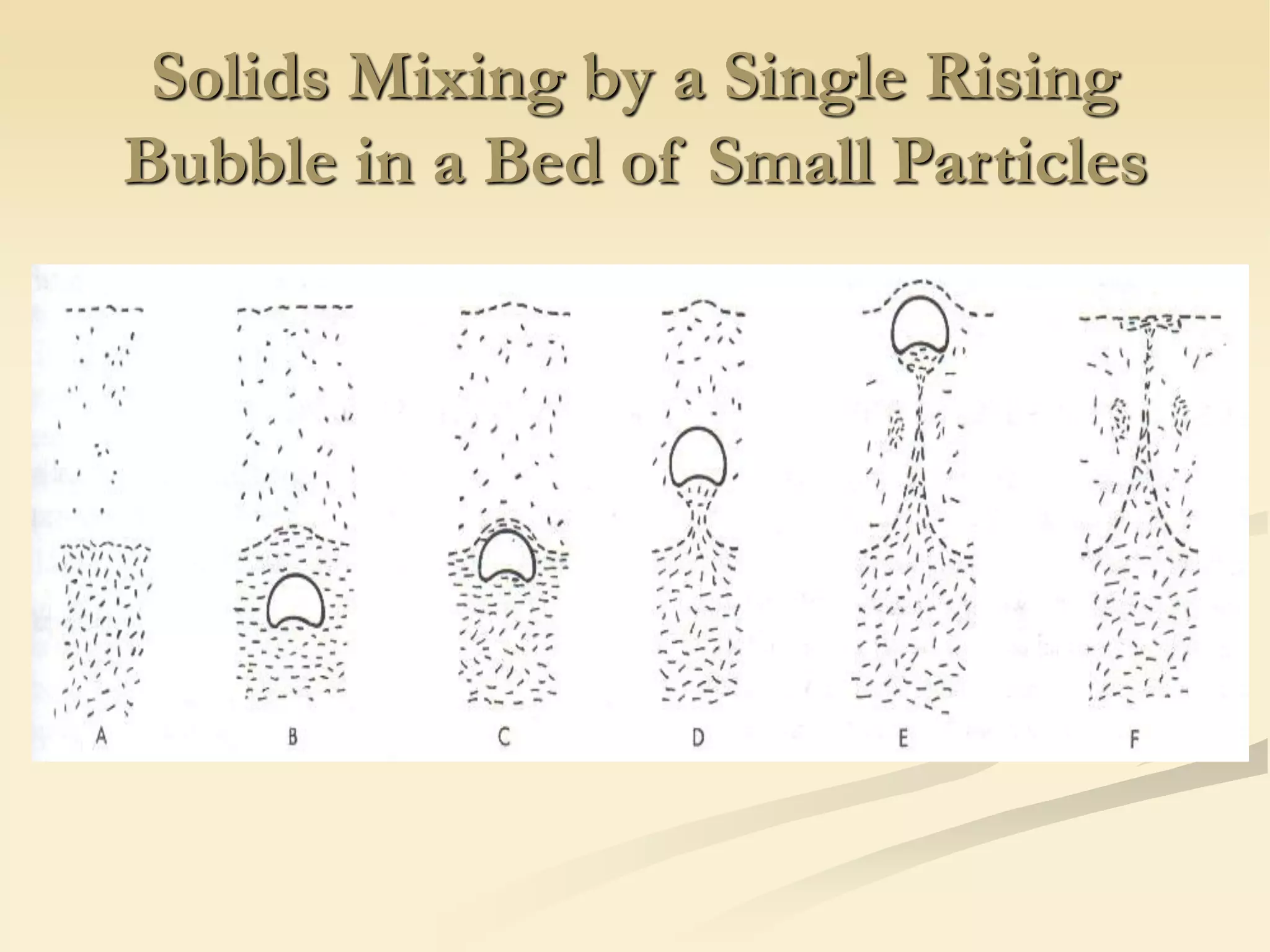
Fluidization is the process of transforming fine solids into a fluid-like state using gas or liquid. It allows for continuous, automatically controlled operations with easy material handling. Fluidized beds provide high rates of heat and mass transfer between gas and particles. Common applications include catalytic reactions, gas-solid reactions like combustion, and physical processes like drying and heat treatment. Proper design of the contacting between phases is important for effectiveness.