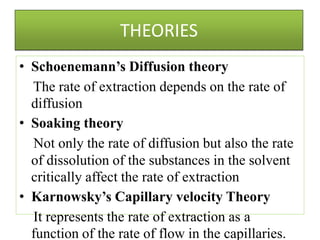
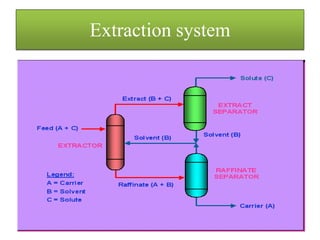
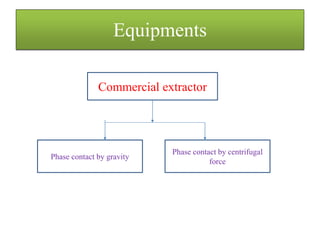
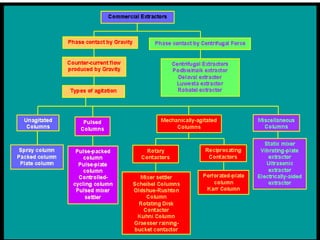
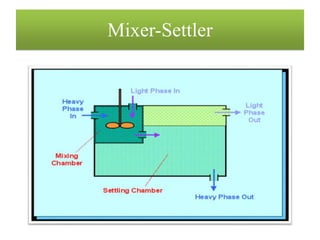
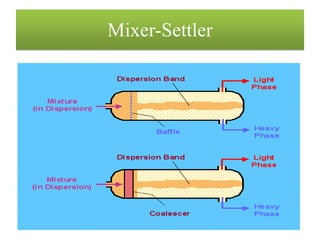
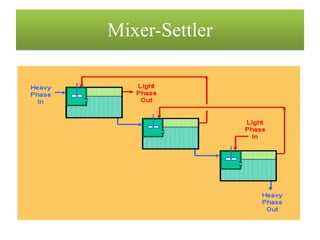
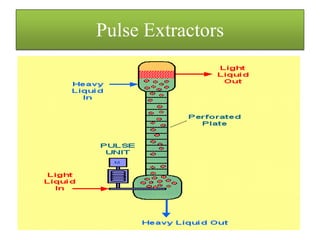
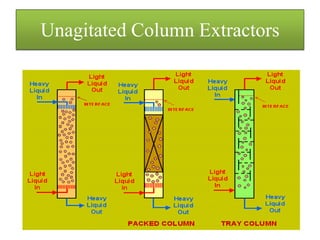
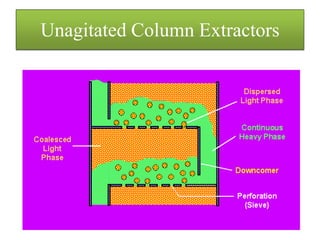
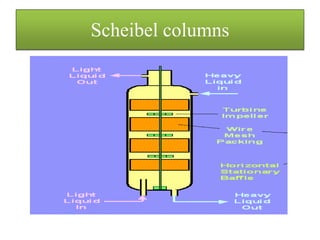
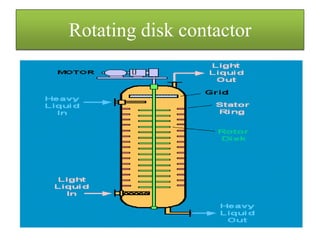
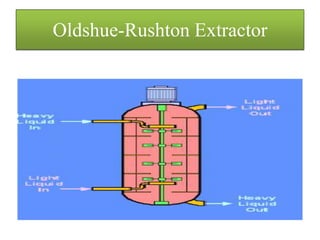
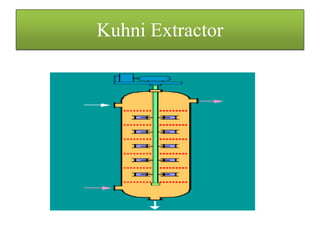
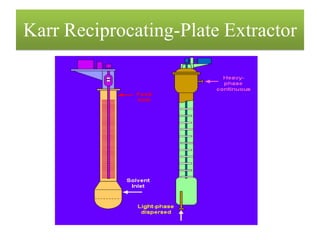
This document discusses extraction and washing processes. It defines extraction as removing solid constituents from solids or liquids using solvents. The solvent dissolves the desired compounds, reaching equilibrium between the solute inside and solvent outside. Rate of extraction depends on factors like mass transfer coefficient, interface area, and solute concentrations. Common extraction methods include liquid-liquid, solid phase, acid-base, supercritical fluid, ultrasound, heat reflux, and microwave extractions. Common equipment used are mixer-settlers, pulse extractors, unagitated columns, and mechanically agitated columns like Scheibel, rotating disk contactor, Oldshue-Rushton, and Kuhni columns. Washing is similar to extraction




![Rate of extraction
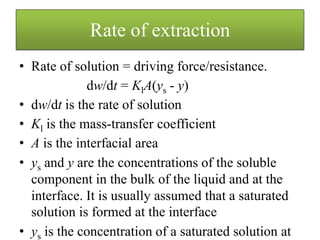
A mass balance on the solute gives the equation
dw = Vdy
V is the quantity of liquid in the liquid stream.
Vdy/dt = Kl A(ys - y)
which can then be integrated over time t during which time the
concentration goes from an initial value of y0 to a concentration
y, giving
loge [(ys - y0)/ (ys - y)] = tKlA/V.](https://image.slidesharecdn.com/extractionandwashing-210217091727/85/Extraction-and-washing-6-320.jpg)



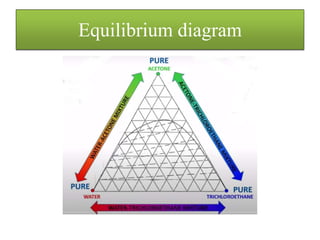
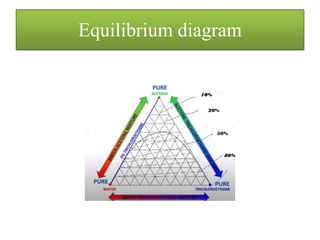
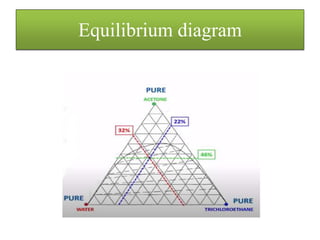

![• The concentration remaining with the solid
after washing
x1 = x[xw /xw(1 + y)] = x[1/(1 + y)]
after two washings:
x2 = x1[1/(1 + y)] = x[1/(1 + y)]2
and so after n washings:
xn = x[1/(1 + y)]n
Washing](https://image.slidesharecdn.com/extractionandwashing-210217091727/85/Extraction-and-washing-33-320.jpg)
