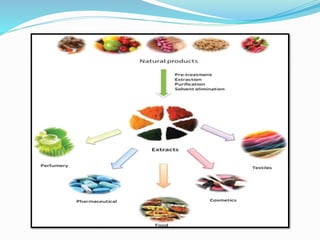
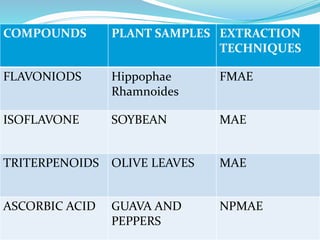
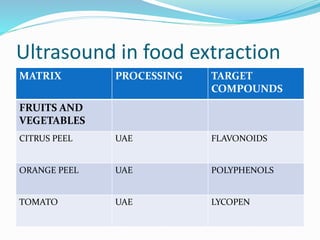
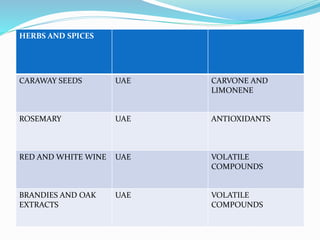
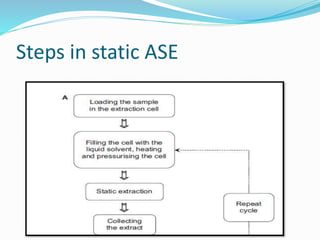
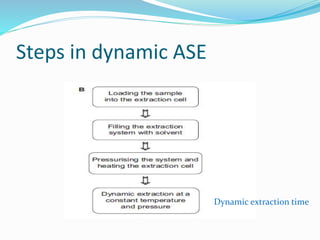
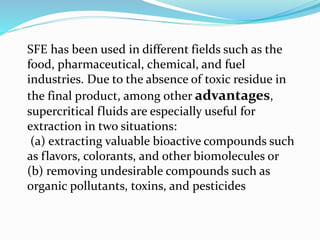
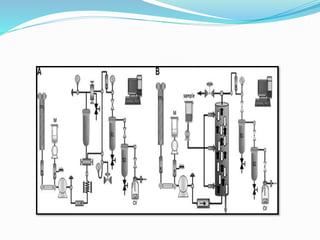
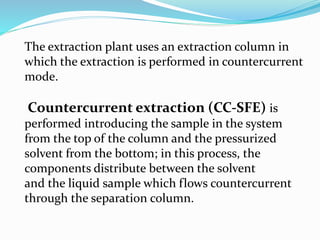
1. Natural products are chemical compounds produced by living organisms found in nature. Extraction is used to separate natural products from plant materials using solvents.
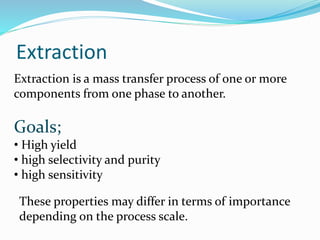
2. Key goals of extraction are high yield, purity, and selectivity. At industrial scales, yield and purity are most important. Extraction depends on properties of the target compound and solvent.
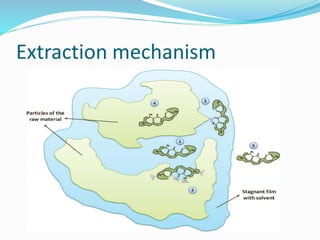
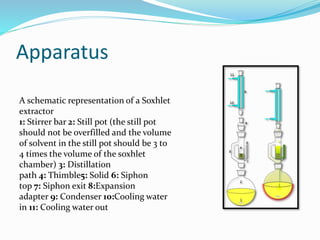
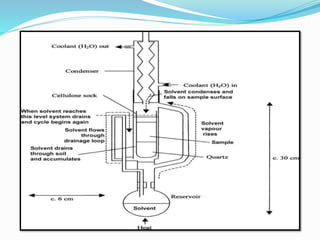
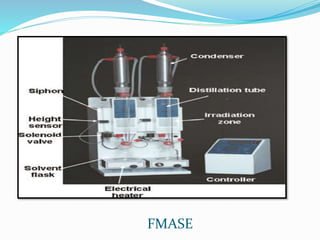
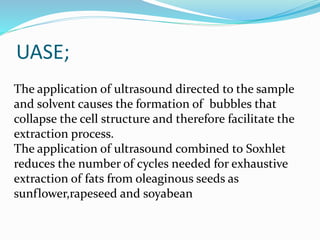
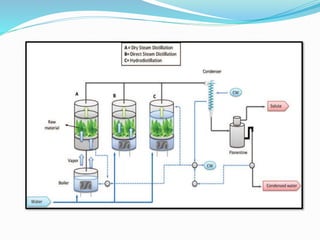
3. Common extraction techniques include soaking, Soxhlet extraction, and distillation. Soxhlet extraction uses repeated cycles of solvent saturated with compound to efficiently separate compounds from plant materials.